2020 Guideline for the Management of Patients with Valvular Heart Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antithrombotic Therapy in Atrial Fibrillation Associated with Valvular Heart Disease
Europace (2017) 0, 1–21 EHRA CONSENSUS DOCUMENT doi:10.1093/europace/eux240 Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardıaca y Electrofisiologıa (SOLEACE) Gregory Y. H. Lip1*, Jean Philippe Collet2, Raffaele de Caterina3, Laurent Fauchier4, Deirdre A. Lane5, Torben B. Larsen6, Francisco Marin7, Joao Morais8, Calambur Narasimhan9, Brian Olshansky10, Luc Pierard11, Tatjana Potpara12, Nizal Sarrafzadegan13, Karen Sliwa14, Gonzalo Varela15, Gemma Vilahur16, Thomas Weiss17, Giuseppe Boriani18 and Bianca Rocca19 Document Reviewers: Bulent Gorenek20 (Reviewer Coordinator), Irina Savelieva21, Christian Sticherling22, Gulmira Kudaiberdieva23, Tze-Fan Chao24, Francesco Violi25, Mohan Nair26, Leandro Zimerman27, Jonathan Piccini28, Robert Storey29, Sigrun Halvorsen30, Diana Gorog31, Andrea Rubboli32, Ashley Chin33 and Robert Scott-Millar34 * Corresponding author. Tel/fax: þ44 121 5075503. E-mail address: [email protected] Published on behalf of the European Society of Cardiology. All rights reserved. VC The Author 2017. For permissions, please email: [email protected]. 2 G.Y.H. Lip 1Institute of Cardiovascular Sciences, University of Birmingham and Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Aalborg University, Denmark (Chair, representing EHRA); 2Sorbonne Universite´ Paris 6, ACTION Study Group, Institut De Cardiologie, Groupe Hoˆpital Pitie´-Salpetrie`re (APHP), INSERM UMRS 1166, Paris, France; 3Institute of Cardiology, ‘G. -

Percutaneous Mitral Valve Therapies: State of the Art in 2020 LA ACP Annual Meeting
Percutaneous Mitral Valve Therapies: State of the Art in 2020 LA ACP Annual Meeting Steven R Bailey MD MSCAI, FACC, FAHA,FACP Professor and Chair, Department of Medicine Malcolm Feist Chair of Interventional Cardiology LSU Health Shreveport Professor Emeritus, UH Health San Antonio [email protected] SRB March 2020 Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company • Grant/Research Support • None • Consulting Fees/Honoraria • BSCI, Abbot DSMB • Intellectual Property Rights • UTHSCSA • Other Financial Benefit • CCI Editor In Chief SRB March 2020 The 30,000 Ft View Maria SRB March 2020 SRB March 2020 Mitral Stenosis • The most common etiology of MS is rheumatic fever, with a latency of approximately 10 to 20 years after the initial streptococcal infection. Symptoms usually appear in adulthood • Other etiologies are rare but include: congenital MS radiation exposure atrial myxoma mucopolysaccharidoses • MS secondary to calcific annular disease is increasingly seen in elderly patients, and in patients with advanced chronic kidney disease. SRB March 2020 Mitral Stenosis • Mitral stenosis most commonly results from rheumatic heart disease fusion of the valve leaflet cusps at the commissures thickening and shortening of the chordae calcium deposition within the valve leaflets • Characteristic “fish-mouth” or “hockey stick” appearance on the echocardiogram (depending on view) SRB March 2020 Mitral Stenosis: Natural History • The severity of symptoms depends primarily on the degree of stenosis. • Symptoms often go unrecognized by patient and physician until significant shortness of breath, hemoptysis, or atrial fibrillation develops. -

How to Define Valvular Atrial Fibrillation?
Archives of Cardiovascular Disease (2015) 108, 530—539 Available online at ScienceDirect www.sciencedirect.com REVIEW How to define valvular atrial fibrillation? Comment définir la fibrillation atriale valvulaire ? ∗ Laurent Fauchier , Raphael Philippart, Nicolas Clementy, Thierry Bourguignon, Denis Angoulvant, Fabrice Ivanes, Dominique Babuty, Anne Bernard Service de cardiologie, faculté de médecine, université Franc¸ois-Rabelais, CHU Trousseau, Tours, France Received 3 June 2015; accepted 8 June 2015 Available online 14 July 2015 KEYWORDS Summary Atrial fibrillation (AF) confers a substantial risk of stroke. Recent trials compar- Atrial fibrillation; ing vitamin K antagonists (VKAs) with non-vitamin K antagonist oral anticoagulants (NOACs) in Valve disease; AF were performed among patients with so-called ‘‘non-valvular’’ AF. The distinction between Stroke ‘‘valvular’’ and ‘‘non-valvular’’ AF remains a matter of debate. Currently, ‘‘valvular AF’’ refers to patients with mitral stenosis or artificial heart valves (and valve repair in North American guidelines only), and should be treated with VKAs. Valvular heart diseases, such as mitral regur- gitation, aortic stenosis (AS) and aortic insufficiency, do not result in conditions of low flow in the left atrium, and do not apparently increase the risk of thromboembolism brought by AF. Post-hoc analyses suggest that these conditions probably do not make the thromboembolic risk less responsive to NOACs compared with most forms of ‘‘non-valvular’’ AF. The pathogenesis of thrombosis is probably different for blood coming into contact with a mechanical prosthetic valve compared with what occurs in most other forms of AF. This may explain the results of the only trial performed with a NOAC in patients with a mechanical prosthetic valve (only a few of whom had AF), where warfarin was more effective and safer than dabigatran. -

Having an Echocardiogram to Screen for a Bicuspid Aortic Valve
Having an echocardiogram to screen for a bicuspid aortic valve UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm What is a bicuspid aortic valve? The aortic valve sits between the main chamber of the heart (the left ventricle) and the aorta. Its function is to ensure that blood flows correctly forward from the left ventricle into the aorta. Normally it has three thin leaflets which open as the heart contracts and then close to prevent back-flow of blood towards the ventricle. Aortic valve Tricuspid aortic valve (normal) Bicuspid aortic valve (abnormal) Some people may be born with an aortic valve made up of only 2 leaflets. The valve is then called bicuspid. This may cause problems with the functioning of the valve in that it may be more prone to gradually becoming narrowed or leaky. Sometimes a bicuspid valve may be associated with widening of the portion of the aorta that is connected to it. Widening of the aorta may occur in some relatives even if they have an aortic valve with 3 leaflets. If this widening is significant it is known as an aortic aneurysm. Is a bicuspid valve a common problem? Studies have suggested that this is not an uncommon valve problem and may occur in up to 1 in 200 people. 2 | PI18_1443_03 Having an echocardiogram to screen for a bicuspid aortic valve Recently there has been evidence that this condition may be genetic and thus have a tendency to run in a family. -

Echo in Asymptomatic Mitral and Aortic Regurgitation
2017 ASE Florida | Orlando, FL October 9, 2017 | 10:40 – 11:00 PM | 20 min | Grand Harbor Ballroom South Echo in Asymptomatic Mitral and Aortic Regurgitation Muhamed Sarić MD, PhD, MPA Director of Noninvasive Cardiology | Echo Lab Associate Professor of Medicine Disclosures Speakers Bureau (Philips, Medtronic) Advisory Board (Siemens) Regurgitation Axioms ▪Typically, regurgitation is NOT symptomatic unless severe ▪The opposite is not true: Severe regurgitation may be asymptomatic ▪ Chronic regurgitation leads to chamber dilatation on either side of the regurgitant valve Regurgitation Discovery ▪ Regurgitation as a anatomic entity was recognized in the 17th century ▪ Regurgitation was first clinically diagnosed by auscultation in the 19th century, well before the advent of echocardiography First Use of Regurgitation Term in English 1683 W. Charleton Three Anat. Lect. i. 18 Those [valves] that are placed in the inlet and outlet of the left Ventricle, to obviate the regurgitation of the bloud into the arteria venosa, and out of the aorta into the left Ventricle. Walter Charleton (1619 – 1707) English Physician Heart Murmur OXFORD ENGLISH DICTIONARY DEFINITION ▪ Any of various auscultatory sounds ▪ Adventitious sounds of cardiac or vascular origin [that is, separate from standard heart sounds: S1, S2, S3, S4] ▪ Sometimes of no significance ▪ But sometimes caused by valvular lesions of the heart or other diseases of the Στῆθος : Stēthos = chest circulatory system René Laënnec Stethoscope (1781 – 1826) (‘Chest examiner’) French Physician Hollow wooden cylinder Inventor of stethoscope in 1816 Laënnec Performing Auscultation Painted by Robert Alan Thom (1915 – 1979), American illustrator Commissioned by Parke, Davis & Co. 1816 1832 René Laënnec, James Hope French physician British physician Invents MONAURAL stethoscope separates MS from MR murmur 1852 1862 George Cammann Austin Flint Sr. -

Avalus™ Pericardial Aortic Surgical Valve System
FACT SHEET Avalus™ Pericardial Aortic Surgical Valve System Aortic stenosis is a common heart problem caused by a narrowing of the heart’s aortic valve due to excessive calcium deposited on the valve leaflets. When the valve narrows, it does not open or close properly, making the heart work harder to pump blood throughout the body. Eventually, this causes the heart to weaken and function poorly, which may lead to heart failure and increased risk for sudden cardiac death. Disease The standard treatment for patients with aortic valve disease is surgical aortic valve Overview: replacement (SAVR). During this procedure, a surgeon will make an incision in the sternum to open the chest and expose the heart. The diseased native valve is then Aortic removed and a new artificial valve is inserted. Once in place, the device is sewn into Stenosis the aorta and takes over the original valve’s function to enable oxygen-rich blood to flow efficiently out of the heart. For patients that are unable to undergo surgical aortic valve replacement, or prefer a minimally-invasive therapy option, an alternative procedure to treat severe aortic stenosis is called transcatheter aortic valve replacement (TAVR). The Avalus Pericardial Aortic Surgical Valve System is a next- generation aortic surgical valve from Medtronic, offering advanced design concepts and unique features for the millions of patients with severe aortic stenosis who are candidates for open- heart surgery. The Avalus Surgical Valve The Avalus valve, made of bovine tissue, is also the only stented surgical aortic valve on the market that is MRI-safe (without restrictions) enabling patients with severe aortic stenosis who have the Avalus valve to undergo screening procedures for potential co-morbidities. -

Congenital Mitral Incompetence and Coarctation Ofaorta
Thorax: first published as 10.1136/thx.27.6.729 on 1 November 1972. Downloaded from Thorax (1972), 27, 729. Congenital mitral incompetence and coarctation of aorta Report of two cases treated surgically' ABDEL K. TERZAKI2, ROBERT D. LEACHMAN3, M. KHALIL ALI, GRADY L. HALLMAN, and DENTON A. COOLEY Department of Medicine and the Cora and Webb Mading Department of Surgery, Baylor University College of Medicine, and the Cardiology and Surgical Sections of the Texas Heart Institute of St. Luke's Episcopal and Texas Children's Hospitals, Houston, Texas, U.S.A. Two patients with congenital mitral incompetence and coarctation of the aorta are presented. One patient had associated patent ductus arteriosus, bicuspid aortic valve, and endocardial fibro- elastosis. The diagnosis in the two patients presented is well established by clinical, laboratory, and surgical findings and also by necropsy examination in one case. It is proposed that the rarity of reported cases in the literature may have resulted from the frequent diagnosis of left ventricular failure in infancy secondary to coarctation, leading to the assumption that a mitral insufficiency murmur, when present, is due to functional regurgitation. Likewise, the murmur may be mistakenly thought to originate from a ventricular septal defect. The diagnosis of coarctation of the aorta presented no problem in either patient, while detection of the mitral incompetence was difficult. Coarctation of the aorta complicated by copyright. pulmonary hypertension in the absence of intracardiac shunt should draw attention to the possi- bility of associated mitral incompetence. Congestive heart failure, especially after correction of coarctation, was also an indication of possible associated mitral insufficiency. -

Prevalence of Migraine Headaches in Patients with Congenital Heart Disease
Prevalence of Migraine Headaches in Patients With Congenital Heart Disease Tam Truong, MD, Leo Slavin, MD, Ramin Kashani, BA, James Higgins, MD, Aarti Puri, BS, Malika Chowdhry, BS, Philip Cheung, BS, Adam Tanious, BA, John S. Child, MD, FAHA, Joseph K. Perloff, MD, and Jonathan M. Tobis, MD* The prevalence of migraine headaches (MH) is 12% in the general population and increases to 40% in patients with patent foramen ovale. This study evaluated the prevalence of MH in patients with congenital heart disease (CHD). Of 466 patients contacted from the UCLA Adult Congenital Heart Disease Center, 395 (85%) completed a questionnaire to determine the prevalence of MH. Patients were stratified by diagnosis of right-to-left, left-to-right, or no shunt. A group of 252 sex-matched patients with acquired cardiovascular disease served as controls. The prevalence of MH was 45% in adults with CHD compared to 11% in the controls (p <0.001). Of the 179 patients with MH, 143 (80%) had migraines with aura and 36 (20%) had migraines without aura versus 36% and 64% observed in the controls (p <0.001). The frequency of MH was 52% in the right-to-left shunt group, 44% in the left-to-right, and 38% in the no ,NS). In patients with a right-to-left shunt who underwent surgical repair ؍ shunt group (p 47% had complete resolution of MH, whereas 76% experienced >50% reduction in headache days per month. In conclusion, the prevalence of MH in all groups of adults with CHD is 3 to 4 times more than a sex-matched control population, with increasing prevalence of MH in patients with no shunt, left-to-right, and right-to-left shunt. -
Heart Failure in Aortic Stenosis — Improving Diagnosis and Treatment Michael R
The new england journal of medicine perspective Heart Failure in Aortic Stenosis — Improving Diagnosis and Treatment Michael R. Zile, M.D., and William H. Gaasch, M.D. The development of heart failure in patients with tional to the stroke volume divided by the square aortic stenosis is associated with a high mortality root of the pressure gradient. Therefore, if the stroke rate — unless aortic-valve replacement is per- volume declines, as it does in some patients with formed. There is an especially high risk of death aortic stenosis in whom heart failure has developed, among patients with aortic stenosis and a decreased there is a proportional decline in the pressure gra- ejection fraction. Before surgery is performed in dient. Under these low-flow conditions, the cal- such patients, initial management must include an culated effective aortic-valve area may indicate the evaluation of the severity of the stenotic lesion and presence of severe aortic stenosis, despite a low the functional state of the left ventricle; in addition, transvalvular pressure gradient. A mean pressure the heart failure must be treated and the patient’s gradient that is less than 30 mm Hg in a patient with condition stabilized. It is possible to pursue both what appears to be severe aortic stenosis (an aortic- of these goals simultaneously with echocardio- valve area of <1 cm2) indicates what is referred to as graphic techniques or cardiac catheterization tech- “low-gradient aortic stenosis.” niques, together with selected pharmacologic in- An example of a low pressure gradient in a pa- terventions. Proper evaluation and treatment require an un- derstanding of the pathophysiology of heart failure ABC in patients with aortic stenosis. -
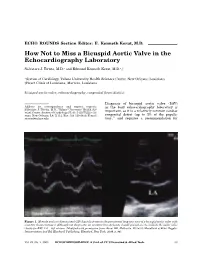
How Not to Miss a Bicuspid Aortic Valve in the Echocardiography Laboratory
ECHO ROUNDS Section Editor: E. Kenneth Kerut, M.D. How Not to Miss a Bicuspid Aortic Valve in the Echocardiography Laboratory Salvatore J. Tirrito, M.D.∗ and Edmund Kenneth Kerut, M.D.∗,† ∗Section of Cardiology, Tulane University Health Sciences Center, New Orleans, Louisiana †Heart Clinic of Louisiana, Marrero, Louisiana bicuspid aortic valve, echocardiography, congenital heart disease Diagnosis of bicuspid aortic valve (BAV) Address for correspondence and reprint requests: in the busy echocardiography laboratory is Salvatore J. Tirrito, M.D., Tulane University Health Sci- important, as it is a relatively common cardiac ences Center, Section of Cardiology SL-48, 1430 Tulane Av- enue, New Orleans, LA 71112. Fax: 504 529-9649; E-mail: congenital defect (up to 3% of the popula- 1 [email protected] tion), and requires a recommendation for Figure 1. M-mode and two-dimensional (2D) diastolic frame in the parasternal long-axis view of a bicuspid aortic valve with eccentric closure (arrows). Although not diagnostic, an eccentric line of closure should prompt one to evaluate the aortic valve closely for BAV. LA = left atrium. (Modified with permission from: Kerut EK, McIlwain, Plotnick: Handbook of Echo-Doppler Interpretation 2nd Ed, Blackwell Publishing, Elmsford, New York, 2004, p. 84) Vol. 22, No. 1, 2005 ECHOCARDIOGRAPHY: A Jrnl. of CV Ultrasound & Allied Tech. 53 TIRRITO AND KERUT Figure 2. Diastolic (left panel) and systolic (right panel) two-dimensional (2D) short-axis image of a BAV. In diastole the raphe may appear as a commissure (arrow), appearing to be a normal tri-leaflet valve. However,the systolic frame has a typical “fish-mouth” appearance. -

How Do We Define Valvular Heart Disease When Considering Warfarin
CORRESPONDENCE Further clarification about this issue would be very much appreciated. Dr Andrew Reid, General Practitioner Tuakau The bpacnz editorial team asked cardiologist Stewart Mann to respond: This is a very relevant question and one that probably does not yet have a definitive answer. The RE-LY trial that compared the efficacy of warfarin and dabigatran had the exclusion criterion “moderate or severe mitral stenosis”. This study also excluded How do we define valvular heart disease when patients with a potentially reversible cause of AF which might considering warfarin or dabigatran in patients with include some valve disease amenable to surgery. Other valve atrial fibrillation? disease could be included. An analysis of patients with valve Dear Editor, disease included in the trial has been conducted and published I have a query from the summary article “An update on in abstract form.1 In the RE-LY trial, around 20% of patients had antithrombotic medications: What does primary care need to valve disease, most with mitral regurgitation but some with know?”, BPJ 73 (Feb, 2016) that I would very much appreciate aortic regurgitation, aortic stenosis or mild mitral stenosis. some further information on. There is no mention of patients with tissue valve prostheses or of those who might have had a valvuloplasty. The group with The article makes this comment: valve disease had poorer outcomes than those without valve “Dabigatran should NOT be prescribed to patients disease but there was no significant difference between those with valvular -

Bicuspid Aortic Valve
© 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Bicuspid Aortic Valve A bicuspid aortic valve is form of congenital heart disease where the aortic valve only has two leaflets, instead of three. This typically results from fusion (joining) of two cusps of the valve along their coaptation point. A bicuspid aortic valve occurs in 1-2% of the population. It can occur alone or be associated with other left-sided heart lesions, such as mitral valve abnormalities (see mitral stenosis), or coarctation of the aorta. Symptoms and presentation can vary for patients with bicuspid aortic valve depending upon the degree of stenosis (narrowing) or regurgitation (leaking) of the valve. Physical Exam/Symptoms: Most patients with bicuspid aortic valve have no symptoms (asymptomatic), unless there is associated aortic stenosis (AS) (narrowing) or regurgitation (leaking) (AR) Most children are asymptomatic with mild to moderate AS. Fatigue, chest pain with exertion, or syncope (fainting) may occur in severe AS. In critical AS, neonates develop poor perfusion, pulmonary edema (fluid retention in the lungs) within days or weeks after birth as the ductus arteriosus (see Patent Ductus Arteriosus) closes. Clinical picture may resemble that of sepsis (severe infection). Murmur of AS: Harsh, grade II/VI systolic murmur heard best at the second left intercostal space, with transmission to the head and neck.