Vacuolar Proton Pumping: More Than the Sum of Its Parts?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix Color Plates of Solanales Species
Appendix Color Plates of Solanales Species The first half of the color plates (Plates 1–8) shows a selection of phytochemically prominent solanaceous species, the second half (Plates 9–16) a selection of convol- vulaceous counterparts. The scientific name of the species in bold (for authorities see text and tables) may be followed (in brackets) by a frequently used though invalid synonym and/or a common name if existent. The next information refers to the habitus, origin/natural distribution, and – if applicable – cultivation. If more than one photograph is shown for a certain species there will be explanations for each of them. Finally, section numbers of the phytochemical Chapters 3–8 are given, where the respective species are discussed. The individually combined occurrence of sec- ondary metabolites from different structural classes characterizes every species. However, it has to be remembered that a small number of citations does not neces- sarily indicate a poorer secondary metabolism in a respective species compared with others; this may just be due to less studies being carried out. Solanaceae Plate 1a Anthocercis littorea (yellow tailflower): erect or rarely sprawling shrub (to 3 m); W- and SW-Australia; Sects. 3.1 / 3.4 Plate 1b, c Atropa belladonna (deadly nightshade): erect herbaceous perennial plant (to 1.5 m); Europe to central Asia (naturalized: N-USA; cultivated as a medicinal plant); b fruiting twig; c flowers, unripe (green) and ripe (black) berries; Sects. 3.1 / 3.3.2 / 3.4 / 3.5 / 6.5.2 / 7.5.1 / 7.7.2 / 7.7.4.3 Plate 1d Brugmansia versicolor (angel’s trumpet): shrub or small tree (to 5 m); tropical parts of Ecuador west of the Andes (cultivated as an ornamental in tropical and subtropical regions); Sect. -

Chloroform Extracts of Ipomoea Alba and Ipomoea Tricolor Seeds Show Strong In-Vitro Antibacterial, Antifungal, and Cytotoxic Activity SIMS K
Research Horizons Day & Research Week April 6-13, 2018 Chloroform Extracts of Ipomoea alba and Ipomoea tricolor Seeds Show Strong In-vitro Antibacterial, Antifungal, and Cytotoxic Activity SIMS K. LAWSON, MARY N. DAVIS, CAROLYN BRAZELL – Biology Department WILLIAM N. SETZER – Mentor – Chemistry Department Overview Antibiotic and antifungal resistance is a growing concern. Novel anti-tumor compounds are continuously sought after. If a novel phytochemical can be discovered with high specificity for certain types of cancer cells, then this could be an invaluable aid to oncological medicine. Plant-based drugs (phyto-pharmaceuticals) have always made up a considerable portion of our known medicines. The search for these plant medicines often begins with anthropological/ ethnobotanical research, as was the case here. Figure 2. - Ancient Olmec tribes mixed the sap of I. alba with sap from the rubber tree to make their rubber balls extra “bouncy”. Their ancient ball games were played since 1300 B.C.. Often, the losers were sacrificed, and sometimes the ball was made from a human Methods skull wrapped in rubber. Figure 1.- Morning glory (Ipomoea spp.) seeds have long been the subject of folklore, myth, and speculation. Some varieties (I. tricolor and I. Cold extractions of the ground seeds of each Ipomoea violacea) contain lysergic acid derivatives, which are known to be species were made with chloroform. Seven bacteria and hallucinogenic, and are closely related chemically to the famous LSD molecule. The Mayans are known to have used morning glory seeds during three fungi were obtained and cultured for multiple certain religious rituals. generations. Then, minimum inhibitory concentrations Table 1.- Antibacterial (MIC, μg/mL), antifungal (MIC, μg/mL), (MIC’s) of the extracts were determined against the and cytotoxic (IC50, μg/mL) activities of Ipomoea CHCl3 seed bacteria and fungi using broth microdilution (BM) extracts. -

Risk Assessment of Argyreia Nervosa
Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos RIVM letter report 2019-0210 Colophon © RIVM 2020 Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited. DOI 10.21945/RIVM-2019-0210 W. Chen (author), RIVM L. de Wit-Bos (author), RIVM Contact: Lianne de Wit Department of Food Safety (VVH) [email protected] This investigation was performed by order of NVWA, within the framework of 9.4.46 Published by: National Institute for Public Health and the Environment, RIVM P.O. Box1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 42 RIVM letter report 2019-0210 Synopsis Risk assessment of Argyreia nervosa In the Netherlands, seeds from the plant Hawaiian Baby Woodrose (Argyreia nervosa) are being sold as a so-called ‘legal high’ in smart shops and by internet retailers. The use of these seeds is unsafe. They can cause hallucinogenic effects, nausea, vomiting, elevated heart rate, elevated blood pressure, (severe) fatigue and lethargy. These health effects can occur even when the seeds are consumed at the recommended dose. This is the conclusion of a risk assessment performed by RIVM. Hawaiian Baby Woodrose seeds are sold as raw seeds or in capsules. The raw seeds can be eaten as such, or after being crushed and dissolved in liquid (generally hot water). -
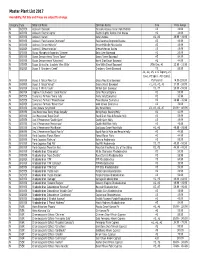
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Gardenergardener®
Theh American A n GARDENERGARDENER® The Magazine of the AAmerican Horticultural Societyy January / February 2016 New Plants for 2016 Broadleaved Evergreens for Small Gardens The Dwarf Tomato Project Grow Your Own Gourmet Mushrooms contents Volume 95, Number 1 . January / February 2016 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS 2016 Seed Exchange catalog now available, upcoming travel destinations, registration open for America in Bloom beautifi cation contest, 70th annual Colonial Williamsburg Garden Symposium in April. 11 AHS MEMBERS MAKING A DIFFERENCE Dale Sievert. 40 HOMEGROWN HARVEST Love those leeks! page 400 42 GARDEN SOLUTIONS Understanding mycorrhizal fungi. BOOK REVIEWS page 18 44 The Seed Garden and Rescuing Eden. Special focus: Wild 12 NEW PLANTS FOR 2016 BY CHARLOTTE GERMANE gardening. From annuals and perennials to shrubs, vines, and vegetables, see which of this year’s introductions are worth trying in your garden. 46 GARDENER’S NOTEBOOK Link discovered between soil fungi and monarch 18 THE DWARF TOMATO PROJECT BY CRAIG LEHOULLIER butterfl y health, stinky A worldwide collaborative breeds diminutive plants that produce seeds trick dung beetles into dispersal role, regular-size, fl avorful tomatoes. Mt. Cuba tickseed trial results, researchers unravel how plants can survive extreme drought, grant for nascent public garden in 24 BEST SMALL BROADLEAVED EVERGREENS Delaware, Lady Bird Johnson Wildfl ower BY ANDREW BUNTING Center selects new president and CEO. These small to mid-size selections make a big impact in modest landscapes. 50 GREEN GARAGE Seed-starting products. 30 WEESIE SMITH BY ALLEN BUSH 52 TRAVELER’S GUIDE TO GARDENS Alabama gardener Weesie Smith championed pagepage 3030 Quarryhill Botanical Garden, California. -

Ipomoea Lacunosa (Convolvulaceae: Ipomoea Sect. Batatas): a Newly Naturalized Species of Taiwan
Taiwan J For Sci 34(1): 55-62, 2019 55 Research note Ipomoea lacunosa (Convolvulaceae: Ipomoea sect. Batatas): a Newly Naturalized Species of Taiwan Chien-Ti Chao,1) Chung-Liang Cheng,2) Chiu-Mei Wang3,4) 【Summary】 Ipomoea sect. Batatas (Choisy) Grisebach is comprised of 11 species mainly distributed in North and South America. Among them, 5 species have been recorded in Taiwan. A few years ago, an unrecognized taxon of Ipomoea was found during our field investigations in central Taiwan. Af- ter consulting the related literature and examining specimens of related species, this unknown plant was confirmed to be I. lacunosa L., which is native to North America. A morphological descrip- tion, photos, and line drawings are provided in this article. The phenology and breeding system of I. lacunosa are also discussed to evaluate the possibility of this species becoming an invasive species in Taiwan in the future. Key words: Convolvulaceae, Ipomoea lacunosa, naturalized species. Chao CT, Cheng CL, Wang CM. 2019. Ipomoea lacunosa (Convolvulaceae: Ipomoea sect. Bata- tas): a newly naturalized species of Taiwan. Taiwan J For Sci 34(1):55-62. 1) Jinhu Elementary School, No. 75, Jinhu Village, Beimen District, Tainan 727, Taiwan. 台南市立錦 湖國民小學,727台南市北門區錦湖里75號。 2) Dr. Cecilia Koo Botanic Conservation Center, 31 Tongxing Rd., Gaoshu Township, Pingtung County 906, Taiwan. 辜嚴倬雲植物保種中心,906屏東縣高樹鄉同興路31號。 3) Department of Biology, National Museum of Natural Science, 1 Guanchien Rd., North District, Taichung 404, Taiwan. 國立自然科學博物館生物學組,404台中市北區館前路1號。 4) Corresponding author, e-mail:[email protected] 通訊作者。 Received January 2018, Accepted September 2018. 2018年1月送審 2018年9月通過。 56 Chao et al.─Ipomoea lacunosa, a newly naturalized species of Taiwan 研究簡報 臺灣旋花科(Convolvulaceae)牽牛花屬蕃薯組(Ipomoea sect. -

Comparative Seed Manual: CONVULVALACEAE Christine Pang, Darla Chenin, and Amber M
Comparative Seed Manual: CONVULVALACEAE Christine Pang, Darla Chenin, and Amber M. VanDerwarker (Completed, June 5, 2019) This seed manual consists of photos and relevant information on plant species housed in the Integrative Subsistence Laboratory at the Anthropology Department, University of California, Santa Barbara. The impetus for the creation of this manual was to enable UCSB graduate students to have access to comparative materials when making in-field identifications. Most of the plant species included in the manual come from New World locales with an emphasis on Eastern North America, California, Mexico, Central America, and the South American Andes. Published references consulted1: 1998. Moerman, Daniel E. Native American ethnobotany. Vol. 879. Portland, OR: Timber press. 2009. Moerman, Daniel E. Native American medicinal plants: an ethnobotanical dictionary. OR: Timber Press. 2010. Moerman, Daniel E. Native American food plants: an ethnobotanical dictionary. OR: Timber Press. Species included herein: Calystegia macrostegia Ipomoea alba Ipomoea amnicola Ipomoea hederacea Ipomoea hederifolia Ipomoea lacunosa Ipomoea leptophylla Ipomoea lindheimeri Ipomoea microdactyla Ipomoea nil Ipomoea setosa Ipomoea tenuissima Ipomoea tricolor Ipomoea tricolor var Grandpa Ott’s Ipomoea triloba Ipomoea wrightii 1 Disclaimer: Information on relevant edible and medicinal uses comes from a variety of sources, both published and internet-based; this manual does NOT recommend using any plants as food or medicine without first consulting a medical professional. Calystegia macrostegia Family: Convulvalaceae Common Names: Island false bindweed, Island morning glory, California morning glory Habitat and Growth Habit: This plant is found in California, the Channel Islands, and Baja California amongst coastal shores, chaparral, and woodlands. Human Uses: Some uses of this species include ornamental/decoration and attraction of hummingbirds. -

Annual Flowers Plant List PM
Last Updated: 5/1/20 9:00 Annual Flowers Plant List PM Please note that any item marked as a 1203 Flat or 1204 Flat consists of twelve 3-packs or twelve 4-packs respectively. FLAT 1204 = Twelve(12) 4-Packs FLAT 1203 = Twelve(12) 3-Packs Plant Series Color/Name Size Abutilon Bella Mix Mix 4" Acalypha Copperhead Copper 4" Agastache Sunrise Blue 4" Agastache Sunrise Orange 4" Agastache Sunrise Red 4" Ageratum Blue FLAT 1204(12-4packs) Alstroemeria Colorita Amina (Orange) 12" Decorative Alstroemeria Colorita Ariane (Yellow) 12" Decorative Alstroemeria Colorita Diana (Yellow Bi Color) 12" Decorative Alstroemeria Colorita Eliane (Pink) 12" Decorative Alstroemeria Colorita Kate (Red) 12" Decorative Alstroemeria Colorita Mulan (Purple Bi Color) 12" Decorative Alteranthera Brazillian Red Hot 4" Alteranthera Little Ruby 4" Alteranthera Red Thread 4" Alyssum Easter Bonnet Formula Mix FLAT 1204(12-4packs) Alyssum Easter Bonnet Pink FLAT 1204(12-4packs) Alyssum Snow Crystal White FLAT 1204(12-4packs) Alyssum Mixed FLAT 1204(12-4packs) Alyssum Pink FLAT 1204(12-4packs) Alyssum White FLAT 1204(12-4packs) Angelonia Angelface Blue 4" Angelonia Angelface Perfectly Pink 4" Angelonia Angelface Wedgewood Blue 4" Angelonia Angelface White 4" Angelonia Archangel Cherry Red 4" Angelonia Archangel Pink 4" Angelonia Serena Blue 4" Angelonia Serena Purple 4" Angelonia Serena Rose 4" Angelonia Serena White 4" Argyranthemum Butterfly Golden Butterfly 4" Argyranthemum Butterfly Pure White 4" Argyranthemum Sassy Double Deep Rose 4" Argyranthemum Sassy Double Yellow -

Ipomeas Background the Genus Ipomoea Consists of Around 500
Ipomeas Background The genus Ipomoea consists of around 500 species of twining vine, bushes or trees from the Convolvulaceae family. Some studies (D. Austin, 1997) have listed between 600 and 700 species, of which over half originate from North and South America. • First there is the Ipomoea purpurea, very well suited to for the temperate climate: it is an American climbing plant whose blue-purple aspect is widely known and which the Americans call Gandpa Ott. The pink flower is also known in Europe. • The ipomoea tricolor (ipomoea tricolor or morning glory) originates from Mexico and Central America. In that region the flower is known as badoh negro and its seeds have been used as hallucinogenics since the Aztec era. It is grown the entire year and puts out a good number of blue flowers – really blue! – of around 10-15 cm. Their highlight is a yellow center. • The ipomoea nil came from Japan some 1,000 years ago from China. At the time it was used as a diuretic; the Chinese had obtained it from the Arabs who traded with all the small kingdoms of East Africa. The Nil arrived accompanied by other Ipomoeas, including one from the Himalayas and another from the Beijing area. They quickly appeared in gardens and were crossed with each other to obtain different colors. They went from being bluish, to pink then white, and are known in Europe as “ipomoea purpurea” and tricolors. One that is much less so is the Japanese ipomoea, “ipomoea nil” or “Asagao”. These flowers found their way into Japanese poetry because they represent a strong theme in Japanese culture: life is short, as is beauty. -

Hallucinogenic Indole Compounds from Higher Plants'
Hallucinogenic Indole Compounds from Higher Plants' ARA DERMARDEROSIAN (Philadelphia College of Pharmacy and Science, Philadelphia, Pennsylvania) In recent years, there has been a revival of interest in the constituents of various species of plants and their uses for therapeutic purposes (12, 21). The literature is replete with examples of chemical and pharmacological studies of numerous plants based on their use in folklore medicine. Extensive literature and field studies of many plants by various ethnic groups have been made. In partic- ular, interesting discoveries have been made of plant sources with some use as psychotherapeutic agents. One of the more specific areas of investigation which has recently received attention, deals with those plants which contain hallucinogenic or psychotomimetic principles. The terms "psychotomimetic" and "hallucinogenic" are used to describe those agents which cause distortion of perception in man, occasionally accompanied by vivid colored illusions and sensations or hallucinations. (The psychotomimetics may be considered as a subdivision of the large class "psycho- tropic", which refers to all substances having some effect on the psyche.) Other names for these substances include psycholytics, psychodysleptics, phantastica, and psychedelics (36, 39, 62, 65, 66). None of these is entirely suitable in describing TABLE 1. Botanical sources of psychotomimetic substances (39). Source and common or native name Active principles ------------------------ ------------------- Leguminosae Piptadenia peregrina Benth. (cohaba) N,N-dimethyltryptamine, bufotenin Piptadenia macrocar-pa Benth. (active doses: 0.05-0.07 g) Malpighiaceae: Banisteriopsis spp. (caapi) harmine, harmaline (active dose: Tetra.pterys spp. (yage, ayahuasca) 0.1-0.4 g) Rutaceae: Peganurn harmala L. harmine, harmaline Agaricaceae: Psilocybe spp. (teonanacatl) psilocybin, psilocin (active dose: Stropharia cubensis Earle 0.006-0.015g) Convolvulaceae: Rivea corymbosa (L.) Hallier f. -

Extraction and Identification of Clavine and Lysergic Acid Alkaloids from Morning Glories1
EXTRACTION AND IDENTIFICATION OF CLAVINE AND LYSERGIC ACID ALKALOIDS FROM MORNING GLORIES1 WELDON L. WITTERS Department of Zoology and Microbiology, Ohio University, Athens, Ohio ^5701 WITTERS, WELDON L. Extraction and alkaloids. These substances are some- identification of clavine and lysergic acid times called ergoline compounds (Der alkaloids from morning glories. Ohio J. Marderosian, 1967). In 1938, Albert Sci. 75(4): 198, 1975. Hofmann of the Sandoz Company, syn- Seeds from three varieties of Ipomoea thesized LSD from ergoline compounds, grown in Ohio were examined for clavine and in 1960 he discovered lysergic acid and lysergic acid alkaloids. Three sys- and clavine alkaloids in morning glory tems of extraction of ergoline alkaloids seeds of Ipomoea violacea Sp. (Hofmann, were compared with the ammonium 1968). hydroxide-methanol-chloroform method A number of investigators, since Hof- proving superior to the others. Four mann, have identified ergoline compounds chromatographic systems were compared in Ipomoea Sp. (Genest, 1966; Niwaguchi for ability to resolve and identify ergoline and Inoue, 1969; Taber et al., 1963). alkaloids. Of the four systems, a thin- The literature records a wide range of layer system with ethyl acetate-ethanol- qualitative and quantitative data on dimethyl-formamide gave the best resolu- ergoline compounds in morning glories. tion of ergoline alkaloids. Ipomoea vio- These data are confused by the variety of lacea L. var. Pearly Gates had signifi- extraction and identification techniques cantly more total alkaloids and more and differences in preparation techniques. individual ergoline alkaloids. In trial runs in our laboratory, it was discovered that quantitative results varied from seed batch to seed batch, For many years, scientists have known and that time and technique of extrac- of a series of hallucinogenic alkaloids in tion and chromatographic separation was such unrelated plants as cactus, mush- very important. -

VINES in the NEW JERSEY LANDSCAPE Barbara J
VINES IN THE NEW JERSEY LANDSCAPE Barbara J. Bromley, Mercer Co. Horticulturist 07 The group considered vines describes a mode of plant behavior, rather than a plant family. Vines are plants whose stems are so weak that they must creep along the ground, hang, or rely on a structure for support. They can be herbaceous or woody, evergreen or deciduous, annual or perennial. As a group, vines are quite versatile and can be used to form a shady bower, screen an unpleasant view or provide colorful flowers and fragrance. They may have showy or edible fruit or enhance an architectural feature. Vines climb by a variety of processes. These include sticky holdfasts, tendrils, weaving or twining on a support, aerial rootlets, and leaning. The growth habit may be dense or open, slow or rapid, contained or invasive. Some vines can cause physical damage to other plants or wooden structures because of their weight or method of clinging. It is important to know the characteristics of a selected plant to know whether it is a good choice for the chosen landscape area or likely to become a headache. Culturally, most vines require a deep, rich, moist, well-drained soil with a pH of about 6.5. Most prefer full sun, but will tolerate partial shade. Many will attach themselves to a trellis or other support, but some have to have hooks provided or need to be attached with twine to hold them in place as they grow. Botanical Name Common name Conditions Flowers Comments Actinidia arguta Tara vine s/ps, moist white Pest resistant.