Molecules and Morphology in South American Stipeae (Poaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -
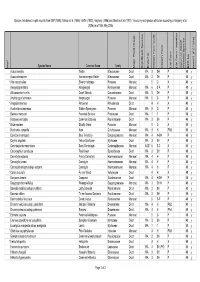
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

GREAT PLAINS REGION - NWPL 2016 FINAL RATINGS User Notes: 1) Plant Species Not Listed Are Considered UPL for Wetland Delineation Purposes
GREAT PLAINS REGION - NWPL 2016 FINAL RATINGS User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps region. -

Poaceae: Pooideae) Based on Plastid and Nuclear DNA Sequences
d i v e r s i t y , p h y l o g e n y , a n d e v o l u t i o n i n t h e monocotyledons e d i t e d b y s e b e r g , p e t e r s e n , b a r f o d & d a v i s a a r h u s u n i v e r s i t y p r e s s , d e n m a r k , 2 0 1 0 Phylogenetics of Stipeae (Poaceae: Pooideae) Based on Plastid and Nuclear DNA Sequences Konstantin Romaschenko,1 Paul M. Peterson,2 Robert J. Soreng,2 Núria Garcia-Jacas,3 and Alfonso Susanna3 1M. G. Kholodny Institute of Botany, Tereshchenkovska 2, 01601 Kiev, Ukraine 2Smithsonian Institution, Department of Botany MRC-166, National Museum of Natural History, P.O. Box 37012, Washington, District of Columbia 20013-7012 USA. 3Laboratory of Molecular Systematics, Botanic Institute of Barcelona (CSIC-ICUB), Pg. del Migdia, s.n., E08038 Barcelona, Spain Author for correspondence ([email protected]) Abstract—The Stipeae tribe is a group of 400−600 grass species of worldwide distribution that are currently placed in 21 genera. The ‘needlegrasses’ are char- acterized by having single-flowered spikelets and stout, terminally-awned lem- mas. We conducted a molecular phylogenetic study of the Stipeae (including all genera except Anemanthele) using a total of 94 species (nine species were used as outgroups) based on five plastid DNA regions (trnK-5’matK, matK, trnHGUG-psbA, trnL5’-trnF, and ndhF) and a single nuclear DNA region (ITS). -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Developing Species-Habitat Relationships: 2016 Project Report
Field Keys to Groups and Alliances in the National Vegetation Classification: Northern Basin & Range / Columbia Plateau Ecoregions NatureServe Conservation Science Division P r i n c i p a l Investigator Patrick J. C o m e r , Chief Ecologist [email protected] 703.797.4802 November 2017 Photos (clockwise from top left; all used under Creative Commons license CC BY 2.0.): Big sage shrubland, Humboldt-Toiyabe National Forest, Nevada. USDA Photo by Susan Elliot. http://flic.kr/p/ax64DY Jeffrey pine woodland, photo by David Prasad. https://www.flickr.com/photos/33671002@N00 Northwest Great Plains Mixedgrass Prairie, Dakota Prairie National Grasslands, North Dakota. Western juniper woodland, BLM Black Hills Recreation Area, Oregon. Acknowledgements This work was completed with funding provided by the Bureau of Land Management through the BLM’s Fish, Wildlife and Plant Conservation Resource Management Program under Cooperative Agreement L13AC00286 between NatureServe and the BLM. Suggested citation: Schulz, K., G. Kittel, M. Reid and P. Comer. 2017. Field Keys to Divisions, Macrogroups, Groups and Alliances in the National Vegetation Classification: Northern Basin & Range / Columbia Plateau Ecoregions. Report prepared for the Bureau of Land Management by NatureServe, Arlington VA. 14p + 58p of Keys + Appendices. See appendix document: Descriptions_NVC_Groups_Alliances_ NorthernBasinRange_Nov_2017.pdf 2 | P a g e Contents Introduction and Background ...................................................................................................................... -

TESIS Caracterización Ambiental De Los Ecosistemas, Zonas De Vida Y
UNIVERSIDAD NACIONAL DANIEL ALCIDES CARRIÓN FACULTAD DE INGENIERÍA ESCUELA DE FORMACIÓN PROFESIONAL DE INGENIERÍA AMBIENTAL TESIS Caracterización Ambiental De Los Ecosistemas, Zonas De Vida Y Vegetación Natural De La Provincia De Pasco Para optar el título profesional de: Ingeniero Ambiental Autor: Bach. Yelitza Orializ VILLEGAS ALANIA Asesor: Ing. Anderson MARCELO MANRIQUE Cerro de Pasco – Perú – 2019 UNIVERSIDAD NACIONAL DANIEL ALCIDES CARRIÓN FACULTAD DE INGENIERÍA ESCUELA DE FORMACIÓN PROFESIONAL DE INGENIERÍA AMBIENTAL TESIS Caracterización Ambiental De Los Ecosistemas, Zonas De Vida Y Vegetación Natural De La Provincia De Pasco Sustentada y aprobada ante los miembros del jurado: Mg. Julio Antonio ASTO LIÑAN Mg. Luis Alberto PACHECO PEÑA PRESIDENTE MIEMBRO Mg. David Johnny CUYUBAMBA ZEVALLOS MIEMBRO 2 DEDICATORIA A mi abuelita, madre y hermanos por el apoyo constante, el empeño y la confianza que me dieron durante el desarrollo y culminación de mis estudios A mi alma mater por brindarme la oportunidad de llegar a ser una profesional 3 RECONOCIMIENTO A Dios por permitirnos tener una buena experiencia dentro de la universidad, por permitir convertimos en profesionales en lo que tanto nos apasiona A mi abuelita, madre y hermanos quienes me brindaron su valiosa y desinteresada orientación y guía en la elaboración del presente trabajo de investigación A cada docente que fue parte de este proceso integral de formación profesional, ya que también fueron un apoyo para poder llegar a esta etapa de nuestras vidas Y a todas las personas que en una u otra forma me apoyaron en la realización de este trabajo 4 RESUMEN La superficie de la Provincia de Pasco abarca dos Eco regiones de las 21 identificadas para nuestro país: La puna de los Andes Centrales y la jalca, las cuales involucran grandes paisajes naturales, con características de formaciones vegetales de matorrales y también de praderas abiertas de gramíneas en los “pajonales” altos andinos. -

The Dry Forests of the Southern Interior Have Been Described As "Fire
Restoration of Ingrown Dry Forests Forest Science Program 2004/05 Annual Technical Report Understory Succession following Ecosystem Restoration of Ingrown Dry Forests FSP Project Number: Y051069 Reg Newman, John Parminter and Sheryl Wurtz April 2005 Restoration of Ingrown Dry Forests Abstract Restoration of ingrown stands of ponderosa pine (Pinus ponderosa) and interior Douglas-fir (Pseudotsuga menziesii var. glauca) was carried out using a prescription of partial cutting and slashing in 1999 and 2000. Partial cutting consisted of thinning the forest canopy and removing intermediate layer trees. Slashing consisted of cutting pre-commercial, intermediate layers to reduce the risk of crown fire during prescribed understory burns. The ponderosa pine stand was subjected to a prescribed fire in April 2004. The partial-cut treatment opened the canopy by 30% at the ponderosa pine site. Three years later, pinegrass production had recovered at a greater rate than most bunchgrasses. Bunchgrass composition had declined in the plant community relative to pinegrass (Calamagrostis rubescens). Total forage standing crop had not yet increased beyond pre-treatment levels. The partial-cut treatment opened the canopy by 27% at the interior Douglas-fir site. Five years later, pinegrass production doubled while bunchgrass remained unchanged. Total forage standing crop increased by about 40%, almost all due to pinegrass increase. A key objective of dry-forest restoration is to increase the abundance of important forage species such as bluebunch wheatgrass (Pseudoroegneria spicata) and rough fescue (Festuca campestris), while reducing the abundance of their primary competitor, pinegrass. It is clear that this objective has not yet been achieved at these two sites. -

Vegetation Classification and Distribution Mapping Report: Hubbell Trading Post National Historic Site
National Park Service U.S. Department of the Interior Natural Resource Program Center Vegetation Classification and Distribution Mapping Report Hubbell Trading Post National Historic Site Natural Resource Technical Report NPS/SCPN/NRTR—2010/301 ON THE COVER Top: Hubbell Trading Post National Historic Site as seen from Hubbell Hill; photo by Courtney White, www.awestthatworks.com. Bottom left: Hubbell Trading Post National Historic Site; photo by Stephen Monroe. Bottom right: Hubbell Wash, photo by Stephen Monroe. Vegetation Classification and Distribution Mapping Report Hubbell Trading Post National Historic Site Natural Resource Technical Report NPS/SCPN/NRTR—2010/301 Authors David Salas Corey Bolen Bureau of Reclamation Remote Sensing and GIS Group Mail Code 86-68211 Denver Federal Center Building 67 Denver, Colorado 80225 Project Manager Anne Cully National Park Service, Southern Colorado Plateau Network P.O. Box 5765 Northern Arizona University Flagstaff, Arizona 86011 Editing and Design Jean Palumbo National Park Service, Southern Colorado Plateau Network P.O. Box 5765 Northern Arizona University Flagstaff, Arizona 86011 March 2010 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituen cies, and the public. The Natural Resource Technical Report Series is used to disseminate results of scientific studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service mission. -

TEMPORAL and SPATIAL VARIATION in ALKALOID LEVELS in Achnatherum Robustum, a NATIVE GRASS INFECTED with the ENDOPHYTE Neotyphodium
Journal of Chemical Ecology, Vol. 32, No. 2, February 2006 ( #2006) DOI: 10.1007/s10886-005-9003-x TEMPORAL AND SPATIAL VARIATION IN ALKALOID LEVELS IN Achnatherum robustum, A NATIVE GRASS INFECTED WITH THE ENDOPHYTE Neotyphodium STANLEY H. FAETH,1,* DALE R. GARDNER,2 CINNAMON J. HAYES,1 ANDREA JANI,1 SALLY K. WITTLINGER,1 and THOMAS A. JONES3 1School of Life Sciences, Arizona State University, PO Box 874501 Tempe, AZ 85287-4501, USA 2USDA<ARS Poisonous Plant Research Laboratory, 1150 East 1400 North, Logan, UT 84341, USA 3USDA<ARS Forage and Range Research Laboratory, Utah State University, Logan, UT 84322, USA (Received August 5, 2005; revised October 4, 2005; accepted October 12, 2005) Published Online March 23, 2006 Abstract—The native North American perennial grass Achnatherum robus- tum (Vasey) Barkworth [= Stipa robusta (Vasey) Scribn.] or sleepygrass is toxic and narcotic to livestock. The causative agents are alkaloidal mycotoxins produced from infections by a systemic and asexual Neo- typhodium endophyte. Recent studies suggest that toxicity is limited across the range of sleepygrass in the Southwest USA. We sampled 17 populations of sleepygrass with varying distance from one focal population known for its high toxicity levels near Cloudcroft, NM, USA. For some, we sampled individual plants twice within the same growing season and over successive years (2001–2004). We also determined infection levels in each population. In general, all populations were highly infected, but infection levels were more variable near the focal population. Only infected plants within populations near the Cloudcroft area produced alkaloids. The ergot alkaloid, ergonovine, comprised the bulk of the alkaloids, with lesser amounts of lysergic and isolysergic acid amides and ergonovinine alkaloids. -

Mechanical and Thermal Properties of Insulating Sustainable Mortars with Ampelodesmos Mauritanicus and Pennisetum Setaceum Plants As Aggregates
applied sciences Article Mechanical and Thermal Properties of Insulating Sustainable Mortars with Ampelodesmos mauritanicus and Pennisetum setaceum Plants as Aggregates Dionisio Badagliacco 1 , Carmelo Sanfilippo 1,*, Bartolomeo Megna 1,2 , Tommaso La Mantia 3 and Antonino Valenza 1 1 Department of Engineering, University of Palermo, 90128 Palermo, Italy; [email protected] (D.B.); [email protected] (B.M.); [email protected] (A.V.) 2 INSTM Research Unity of Palermo, 90128 Palermo, Italy 3 Department of Agricultural, Food and Forest Sciences, University of Palermo, 90128 Palermo, Italy; [email protected] * Correspondence: carmelo.sanfi[email protected] Abstract: The use of natural fibers in cement composites is a widening research field as their application can enhance the mechanical and thermal behavior of cement mortars and limit their carbon footprint. In this paper, two different wild grasses, i.e., Ampelodesmos mauritanicus, also called diss, and Pennisetum setaceum, also known as crimson fountaingrass, are used as a source of natural aggregates for cement mortars. The main purpose is to assess the possibility of using the more invasive crimson fountaingrass in place of diss in cement-based vegetable concrete. The two plant fibers have been characterized by means of scanning electron microscopy (SEM), helium Citation: Badagliacco, D.; Sanfilippo, picnometry and thermogravimetric analysis. Moreover, the thermal conductivity of fiber panels has C.; Megna, B.; La Mantia, T.; Valenza, been measured. Mortars samples have been prepared using untreated, boiled and Polyethylene glycol A. Mechanical and Thermal 4000 (PEG) treated fibers. The mechanical characterization has been performed by means of three Properties of Insulating Sustainable point bending and compression tests. -

Poaceae) Author(S): Raúl Gonzalo , Carlos Aedo , and Miguel Ángel García Source: Systematic Botany, 38(2):344-378
Taxonomic Revision of the Eurasian Stipa Subsections Stipa and Tirsae (Poaceae) Author(s): Raúl Gonzalo , Carlos Aedo , and Miguel Ángel García Source: Systematic Botany, 38(2):344-378. 2013. Published By: The American Society of Plant Taxonomists URL: http://www.bioone.org/doi/full/10.1600/036364413X666615 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Systematic Botany (2013), 38(2): pp. 344–378 © Copyright 2013 by the American Society of Plant Taxonomists DOI 10.1600/036364413X666615 Taxonomic Revision of the Eurasian Stipa Subsections Stipa and Tirsae (Poaceae) Rau´ l Gonzalo,1,2 Carlos Aedo,1 and Miguel A´ ngel Garcı´a1 1Real Jardı´n Bota´nico, CSIC, Dpto. de Biodiversidad y Conservacio´n. Plaza de Murillo 2, 28014 Madrid, Spain. 2Author for correspondence ([email protected]) Communicating Editor: Lucia G. Lohmann Abstract—A comprehensive taxonomic revision of Stipa subsects.