Pharmaceutical Price Regulation Scheme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chief Executive Officer Graham Baker
OVERVIEW OUR BUSINESS OPERATIONAL FINANCIAL RISK GOVERNANCE ACCOUNTS 48 & MARKETPLACE REVIEW REVIEW OUR BOARD OF DIRECTORS ROBERTO QUARTA (67) OLIVIER BOHUON (58) GRAHAM BAKER (48) CHAIRMAN CHIEF EXECUTIVE OFFICER CHIEF FINANCIAL OFFICER Joined the Board in December 2013 and Joined the Board and was appointed Chief -RLQLQJWKH%RDUGDV&KLHI)LQDQFLDO2I²FHULQ appointed Chairman following election by ([HFXWLYH2I²FHULQ$SULO+HUHVLJQHGDV March 2017. shareholders at the April 2014 Annual General a Member of the Nomination & Governance Meeting. He was also appointed Chairman of &RPPLWWHHRQ)HEUXDU\ CAREER AND EXPERIENCE the Nomination & Governance Committee and Graham holds an MA degree in Economics from a Member of the Remuneration Committee on CAREER AND EXPERIENCE Cambridge University and qualified as a Chartered that day. Olivier holds a doctorate in Pharmacy from the Accountant and Chartered Tax Advisor with Arthur University of Paris and an MBA from HEC, Paris. Andersen. In 1995, he joined AstraZeneca PLC where CAREER AND EXPERIENCE He started his career in Morocco with Roussel Uclaf he worked for 20 years, holding multiple senior roles, Roberto is a graduate and a former Trustee of the S.A. and then, with the same company, held a number including Vice President, Finance, International (2013- College of the Holy Cross, Worcester (MA), US. of positions in the Middle East with increasing levels of 2015) with responsibility for all emerging markets, He started his career as a manager trainee at David responsibility. He joined Abbott in Chicago as head of Vice President, Global Financial Services (2011-2013) Gessner Ltd, before moving on to Worcester Controls their anti-infective franchise with Abbott International and Vice President Finance & Chief Financial Officer, Corporation and then BTR plc, where he was a before becoming Pharmaceutical General Manager North America (2008-10). -

1999 Annual Report and Form 20-F CONTENTS
1999 Annual Report and Form 20-F CONTENTS Key Achievements in 1999 1 Financial Highlights 2 Shareholder Highlights 5 Chairman’s Statement 6 Chief Executive’s Review 7 Operational Review 8 Safety, Health and Environment 32 In April 1999, Astra AB and Zeneca Group PLC People and Community 34 merged to form AstraZeneca, one of the world’s Financial Review 35 leading pharmaceutical and agrochemical Board of Directors and Officers companies, which provides innovative, effective of the Company 48 products to improve health, nutrition and quality Directors’ Report 49 of life worldwide. The company is research and Financial Statements 55 technology intensive, with extensive international Financial statements and notes development and marketing skills. Its healthcare relating to the financial statements 55 business is strategically focused on seven major Principal subsidiaries, joint ventures therapeutic areas: gastrointestinal, oncology, pain and associates 118 control and anaesthesia, cardiovascular, central Additional information for US investors 120 nervous system, respiratory and infection. Zeneca Group Financial Record 129 Agrochemicals provides crop protection products Shareholder Information 131 designed to improve crop yields and food quality. Exchange rates 138 Definitions 139 Glossary of Terms 140 Cross Reference to Form 20-F IBC Cautionary statement regarding forward-looking statements In order to utilise the ‘Safe Harbor’ provisions of the United States Private Securities Litigation Reform Act of 1995, AstraZeneca is providing the following cautionary statement. This Annual Report and Form 20-F 1999 contains forward-looking statements with respect to the financial condition, results of operations and businesses of AstraZeneca. By their nature, forward-looking statements and forecasts involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. -

Astrazeneca Notice of AGM 2007
NOTICE OF ANNUALAND SUMMARY GENERAL MEETINGFINANCIAL STATEMENT 2007 AND SHAREHOLDERS’ cIRCULAR WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd LETTER FROM THE CHAIRMAN 1 LETTER FROM THE CHAIRMAN THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE AttENTION. IF YOU ARE or incurring because of the Political Parties, IN ANY DOUBT ABOUT ITS CONTENTS OR WHAT ACTION YOU SHOULD TAKE, YOU SHOULD Elections and Referendums Act 2000 in the CONSULT YOUR INDEPENDENT FINANCIAL ADVISER. IF YOU HAVE SOLD OR TRANSFERRED ALL UK (“the Act”). OF YOUR ASTRAZENECA ORDINARY SHARES YOU SHOULD SEND THIS DOCUMENT AND THE ACCOMPANYING DOCUMENTS TO THE PURCHASER OR TRANSFEREE OR TO THE STOCKBROKER, The Company has no intention of changing its BANK OR OTHER AGENT THROUGH WHOM THE SALE OR TRANSFER WAS EFFECTED FOR current practice of not making donations to TRANSMISSION TO THE PURCHASER OR TRANSFEREE. political parties in the EU and it will not do so without the specific endorsement of its shareholders. However, the Act defines DEAR SHAREHOLDER Senior Independent Director. Sir Peter was ‘political organisation’ widely so as to include, On behalf of the Board of AstraZeneca PLC, first appointed to the Board in January 1995. amongst other things, an organisation that I enclose various documents concerning your carries on activities that are capable of being shareholding in the Company. These are: On behalf of the whole Board, I would like reasonably regarded as intended to influence to express our gratitude to Erna and Sir public support for a political party in any EU 1 A Shareholders’ Circular incorporating Peter for their considerable contribution to member state or to influence voters in relation the formal Notice of the Annual General AstraZeneca’s success and wish them both to any referendum in any EU member state. -
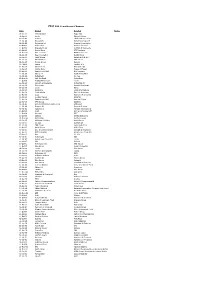
FTSE 100 Constituent History Updated
FTSE 100 Constituent Changes Date Added Deleted Notes 19-Jan-84 CJ Rothschild Eagle Star 02-Apr-84 Lonrho Magnet Sthrns. 02-Jul-84 Reuters Edinburgh Inv. Trust 02-Jul-84 Woolworths Barrat Development 19-Jul-84 Enterprise Oil Bowater Corporation 01-Oct-84 Willis Faber Wimpey (George) 01-Oct-84 Granada Group Scottish & Newcastle 01-Oct-84 Dowty Group MFI Furniture 04-Dec-84 Brit. Telecom Matthey Johnson 02-Jan-85 Dee Corporation Dowty Group 02-Jan-85 Argyll Group Berisford (S.& W.) 02-Jan-85 MFI Furniture RMC Group 02-Jan-85 Dixons Group Dalgety 01-Feb-85 Jaguar Hambro Life 01-Apr-85 Guinness (A) Enterprise Oil 01-Apr-85 Smiths Inds. House of Fraser 01-Apr-85 Ranks Hovis McD. MFI Furniture 01-Jul-85 Abbey Life Ranks Hovis McD. 01-Jul-85 Debenhams I.C. Gas 06-Aug-85 Bnk. Scotland Debenhams 01-Oct-85 Habitat Mothercare Lonrho 02-Jan-86 Scottish & Newcastle Rothschild (J) 08-Jan-86 Storehouse Habitat Mothercare 08-Jan-86 Lonrho B.H.S. 01-Apr-86 Wellcome EXCO International 01-Apr-86 Coats Viyella Sun Life Assurance 01-Apr-86 Lucas Harrisons & Crosfield 01-Apr-86 Cookson Group Ultramar 21-Apr-86 Ranks Hovis McD. Imperial Group 22-Apr-86 RMC Group Distillers 01-Jul-86 British Printing & Comms. Corp Abbey Life 01-Jul-86 Burmah Oil Bank of Scotland 01-Jul-86 Saatchi & S. Ferranti International 01-Oct-86 Bunzl Brit. & Commonwealth 01-Oct-86 Amstrad BICC 01-Oct-86 Unigate Smiths Industries 09-Dec-86 British Gas Northern Foods 02-Jan-87 Hillsdown Holdings Argyll Group 02-Jan-87 I.C. -

Astrazeneca Annual Report 2005
AstraZeneca Annual Report and Form 20-F Information 2005 Annual Report and Form 20-F Information 2005 DRIVING PROGRESS THROUGH PERFORMANCE CONTENTS 2005 in Brief 1 Financial Statements 81 21. Dividends to shareholders 106 Chairman’s Statement 2 Preparation of the Financial Statements 22. Disposal of business operations 106 Chief Executive’s Review 3 and Directors’ Responsibilities 82 23. Post-retirement benefits 107 Financial Highlights 5 Basis of Consolidation and Presentation 24. Employee costs and share option of Financial Information 82 Business Review 6 plans for employees 111 Independent Auditors’ Report to the Business Environment 7 25. Commitments and Members of AstraZeneca PLC (Group) 83 contingent liabilities 116 Strategy 9 Consolidated Income Statement 84 26. Leases 126 Delivering Strategy 10 Consolidated Statement 27. Statutory and other information 127 Measuring Performance 12 of Recognised Income and Expense 84 28. Share capital of parent company 128 Reporting Performance 13 Consolidated Balance Sheet 85 Principal Subsidiaries 129 Therapy Area Review Consolidated Cash Flow Statement 86 Additional Information for US Investors 130 Cardiovascular Medicines 14 Accounting Policies (Group) 87 Explanation of Transition to IFRS 137 Gastrointestinal Medicines 18 Notes to the Financial Statements (Group) 90 Independent Auditors’ Report to the Neuroscience Medicines 21 1. Operating profit 90 Members of AstraZeneca PLC (Company) 139 Oncology Medicines 24 2. Profit on sale of interest in joint venture 90 Company Balance Sheet 140 Respiratory and 3. Finance income and expense 90 Accounting Policies (Company) 141 Inflammation Medicines 27 4. Taxation 91 Notes to the Financial Infection Medicines 30 5. Earnings per $0.25 Ordinary Share 93 Statements (Company) 142 Geographic Review 31 6. -
Client List LATEST
Client number client name country area code client comments corporate number 1382 3 FHEA LAB AD GER SONER C11612 1017 3M MEDICAL DEPARTMENT USA ST PAUL 785 A.H. MARNE & CO LTD UK BRADFORD C11634 1781 ART JAPAN INC. JAP TOKYO 103 Applied Ananlytical Immagary Inc. 644 ABBOTT LABORATORIES LTD US ilILLINOIS Abbott Park C10042 1789 ACTIVE BIOTECH RESEARCH SWE LUND Lund Research Centre AB 1244 AGAN CHEMICAL ITA C10693 1245 AGOURON PHARMACEUTICALS INC. USA SAN DIEGO C10007 774 AGREVO UK LTD UK Now use 1849 Aventis CropScience 1302 AGRITECH UK LIMITED UK SELBOURNE C11646 619 AGRO KAHEAHO COMPANY LTD JAP TOKYO C10854 1773 AIR PRODUCTS AND CHEMICALS INC USA USA C10007 702 AJIMOMOTO CO INC JAP C10323 1946 AJIMOMOTO PHARMACEUTICALS EURO UK REDHILL EUROPE LTD 1043 AKED INTERVET NET C10211 1710 AKZO NOBEL SWE STENLUNGEUN C10210 254 ALBRIGHT & WILSON UK LIMITED UK HENLEY C11661 ALEEN LABORATORIES INC USA FORT WORTH C10663 1747 ALIZYME THERAPEUTICSLTD UK CAMBRIDGE C14475 ALLELEX BIO PHARMACEUTICALS CAN ONTARIO C14103 804 ALLERMAN UK C10903 2791 ALLERCENE LTD RAMAT ISRAEL 1725 ALLERGY THERAPEUTICS LTD UK WORTHING 635 ALMIRALL SPA BARCELONA C10320 1741 ALPHARMA AU NOR OSLO C23276 628 AMERICAN CYANAMID COMPANY USA C10016 1838 AMRAD OPERATIONS PTY LTD AUS RICHMOND C10135 1702 AMVAC CHEMICAL CORPORATIONUSA USA CITY OF CO City of COMMERCE, California C10814 1184 AMYLIN EUROPE LTD UK OXFORD C10020 1070 ANCARE NEW ZEALAND LTD NZ AUCKLAND 607 ANT. INTERNATIONAL LTD UK SUDBURY C11694 1622 ANTISEPTICA GmbH GER 1070 AORTECH EUROPE LTD UK LEEDS 1098 APHTON CORPORATION USA WOODLAND CITY C10916 1737 APOTEX RESEARCH INC. CAN ONTARIO C14502 1628 AQUAMARINE (LONDON) LTD UK HOUNSLOW 1711 ARIAD PHARMACEUTICALS INC USA MASS. -
FTSE 100 Historic Additions and Deletions
FTSE 100 Historic Additions and Deletions ftserussell.com An LSEG Business September 2021 FTSE 100 – Historic Additions and Deletions Date Added Deleted Notes 19-Jan-84 Charterhouse J Rothschild Eagle Star Corporate Event - Acquisition of Eagle Star by BAT Industries 02-Apr-84 Lonrho Magnet & Southerns 02-Jul-84 Reuters Edinburgh Investment Trust 02-Jul-84 Woolworths Barratt Development 19-Jul-84 Enterprise Oil Bowater Corporation Corporate Event - Sub division of company into Bowater Inds and Bowater Inc 01-Oct-84 Willis Faber Wimpey (George) & Co 01-Oct-84 Granada Group Scottish & Newcastle Breweries 01-Oct-84 Dowty Group MFI Furniture Corporate Event - Acquisition of MFI Furniture by Associated Dairies Group 04-Dec-84 British Telecom Johnson Matthey Fast Entry 02-Jan-85 Dee Corporation Dowty Group 02-Jan-85 Argyll Group Berisford (S & W) 02-Jan-85 MFI Furniture RMC Group 02-Jan-85 Dixons Group Dalgety 01-Feb-85 Jaguar Hambro Life Corporate Event - Acquisition of Hambro Life by BAT Industries 01-Apr-85 Guinness (Arthur) & Son Enterprise Oil 01-Apr-85 Smith Industries House of Fraser Corporate Event - Acquisition of House of Fraser by Alfayed Investment Trust 01-Apr-85 Ranks Hovis McDougall MFI Furniture Corporate Event - Acquisition of MFI Furniture by Associated Dairies Group 01-Jul-85 Abbey Life Ranks Hovis McDougall 01-Jul-85 Debenhams Imperial Continental Gas Ass. 06-Aug-85 Bank of Scotland Debenhams 01-Oct-85 Habitat Mothercare Lonrho 02-Jan-86 Scottish & Newcastle Rothschild (J) Holdings 08-Jan-86 Storehouse Habitat Mothercare -
Annual Report on Form 20-F 2018
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 20-F (Mark One) ☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 or ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2018 Or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Or ☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Date of event requiring this shell company report For the transition period from to Commission File Number: 001-31368 Sanofi (Exact name of registrant as specified in its charter) N/A (Translation of registrant’s name into English) France (Jurisdiction of incorporation or organization) 54, Rue La Boétie, 75008 Paris, France (Address of principal executive offices) Karen Linehan, Executive Vice President Legal Affairs and General Counsel 54, Rue La Boétie, 75008 Paris, France. Fax: 011 + 33 1 53 77 43 03. Tel: 011 + 33 1 53 77 40 00 (Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person) Securities registered or to be registered pursuant to Section 12(b) of the Act: Title of each class: Name of each exchange on which registered: American Depositary Shares, each representing one half of one ordinary share, par value€2 per share NASDAQ Global Select Market Ordinary shares, par value€2 per share NASDAQ Global Select Market* Contingent Value Rights NASDAQ Global Market Securities registered pursuant to Section 12(g) of the Act: None The number of outstanding shares of each of the issuer’s classes of capital or common stock as of December 31, 2018 was: Ordinary shares: 1,245,454,385 Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. -

2007English.87123248
ASTRAZENECA CONTACT ANNUAL REPORT AND INFORMATION FORM 20-F INFORMATION 2007 REGISTERED OFFICE INVESTOR RELATIONS REGISTRAR AND US DEPOSITARY AND CORPORATE CONTACTS TRANSFER OFFICE JPMorgan Chase Bank HEADQUARTERS ADDRESS UK: as above or e-mail Equiniti Limited JPMorgan Service Center AstraZeneca PLC [email protected] Aspect House PO Box 3408 15 Stanhope Gate Sweden: Spencer Road South Hackensack London W1K 1LN AstraZeneca AB Lancing NJ 07606-3408 UK SE-151 85 Södertälje West Sussex US Tel: +44 (0)20 7304 5000 Sweden BN99 6DA Tel (toll free in the US): Fax: +44 (0)20 7304 5151 Tel: +46 (0)8 553 260 00 UK 888 697 8018 Fax: +46 (0)8 553 290 00 Tel (freephone in the UK): Tel (outside the US): or e-mail 0800 389 1580 +1 (201) 680 6630 [email protected] Tel (outside the UK): US: +44 (0)121 415 7033 Investor Relations AstraZeneca SWEDISH SECURITIES ASTRAZENECA.COM Pharmaceuticals LP REGISTRATION CENTRE 1800 Concord Pike VPC AB PO Box 15437 PO Box 7822 Wilmington SE-103 97 Stockholm DE 19850-5437 Sweden US Tel: +46 (0)8 402 9000 Tel: +1 (302) 886 3000 Fax: +1 (302) 886 2972 2007 AND FORM 20-F INFORMATION ANNUAL REPORT ASTRAZENECA This Annual Report and Form 20-F Information is also available online at astrazeneca.com/ annualreport2007 INTRODUCTION 01 > International accounting transition 92 26 Employee costs and share option plans for employees 153 AstraZeneca and our year in brief 01 > New accounting standards 92 > Sarbanes-Oxley Act section 404 92 27 Commitments and Financial highlights 02 contingent liabilities 158 > Results of operations -

The Influence of the Pharmaceutical Industry
House of Commons Health Committee The Influence of the Pharmaceutical Industry Fourth Report of Session 2004–05 Volume I Report, together with formal minutes Ordered by The House of Commons to be printed 22 March 2005 HC 42-I [Incorporating HC 1030-i-iii] Published on 5 April 2005 by authority of the House of Commons London: The Stationery Office Limited £15.50 The Health Committee The Health Committee is appointed by the House of Commons to examine the expenditure, administration, and policy of the Department of Health and its associated bodies. Current membership Mr David Hinchliffe MP (Labour, Wakefield) (Chairman) Mr David Amess MP (Conservative, Southend West) John Austin MP (Labour, Erith and Thamesmead) Mr Keith Bradley MP (Labour, Manchester Withington) Mr Simon Burns MP (Conservative, Chelmsford West) Mrs Patsy Calton MP (Liberal Democrat, Cheadle) Jim Dowd MP (Labour, Lewisham West) Mr Jon Owen Jones MP (Labour, Cardiff Central) Siobhain McDonagh MP (Labour, Mitcham and Morden) Dr Doug Naysmith MP (Labour, Bristol North West) Dr Richard Taylor MP (Independent, Wyre Forest) Powers The Committee is one of the departmental select committees, the powers of which are set out in House of Commons Standing Orders, principally in SO No 152. These are available on the Internet via www.parliament.uk. Publications The Reports and evidence of the Committee are published by The Stationery Office by Order of the House. All publications of the Committee (including press notices) are on the Internet at www.parliament.uk/parliamentary_committees/health_committee.cfm A list of Reports of the Committee in the present Parliament is at the back of this volume. -

Organization Name Add1 Add2 Add3 City A. Menarini Diagnostics Ltd Wharfedale Road Winnersh Wokingham Berkshire A1 Pharmaceutical
Organization Name Add1 Add2 Add3 City A. Menarini Diagnostics Ltd Wharfedale Road Winnersh Wokingham Berkshire A1 Pharmaceuticals Plc Units 3/4 Bessemer Park Ind.Est 250 Milkwood Road Herne Hill London AAF Bassington Lane Cramlington Northumbeland AAH Medical Unit 20 - Broombank Business Park Broombank Rd. Sheepbridge Chesterfield Derbyshire AAH Pharmaceuticals Ltd Walsgrave Triangle Walsgrave Coventry West Midlands Abbott Laboratories Limited Abbott House Norden Road Maidenhead Berks Aber Instruments Ltd Science Park Aberystwyth Aberystwyth Abertec Ltd The Old College Kings Street Aberystwyth Aberystwyth abtec Biologicals Ltd 396 Scotland Road Taylor Street I E Liverpool Merseyside Acambis PLC Peterhouse Technology Park 100 Fulbourn Road Cambridge Cambridgeshire Actinova Ltd 5 Signet Court Swanns Road Cambridge Cambs Adams Healthcare Ltd Lotherton Way Garforth Leeds Adaptive Biosystems Ltd 15 Ribocon Way Progress Business Park Luton Beds Adelphi (Tubes) Ltd Millgreen Road Haywards Heath West Sussex Advanced Biotechnologies Ltd Unit Units B1 & B2 Longmead Business Centre Blenheim Road Surrey Epsom Advanced Medical Solutions Group Plc Road Three Winsford Industrial Estate Windsford Cheshire Advanced Technologies (Cambridge) Ltd (ATC) 210 Cambridge Science Park Milton Road Cambridge Cambs Advante Holdings (UK) Ltd Plant Breeding Station Boothby Graffoe Lincs Aea Technology Biotechnology Services 353 Harwell Didcot Oxfordshire AEA Technology plc AEA Technology Biosciences F4 Culham Science Centre Abingdon Oxon AERES Biomedical Ltd 1-3 Burtonhole -
Meddelande Om Astrazenecas Styrelse
2004-12-17 14:02 CET Meddelande om AstraZenecas styrelse AstraZeneca bekräftar idag utnämningen av Louis Schweitzer som ordförande i AstraZenecas styrelse (Non-Executive Chairman) från och med den 1 januari 2005. Percy Barnevik kommer att avgå från styrelsen och ordförandeposten den 31 december 2004. Utnämningen av Louis Schweitzer som ordförande sker som förväntat efter att han valts in i styrelsen den 11 mars 2004. AstraZeneca meddelar samtidigt att dr John Patterson utnämns till styrelseledamot och Excecutive Director från och med den 1 januari 2005 med ansvar för Development. Martin Nicklasson blir Executive Vice-President, Product Strategy and Licensing samt VD för AstraZeneca AB. Kontaktperson: Staffan Ternby, 08-553 261 07 Biografier Louis Schweitzer AstraZeneca PLC announced the appointment of Louis Schweitzer as a non-executive Director of the company on March 11 2004. He was elected Chairman and Chief Executive Officer of Renault in May 1992. Since taking office, Mr. Schweitzer has opened the company to private shareholders in 1994 and led the privatization of Renault in 1996. On March 27, 1999, Renault and Nissan agreed to join forces to achieve profitable growth for both companies forming a new entity ranking fifth in the world automotive industry. Since then, Renault has taken a majority stake in Dacia and Samsung in 2000 and became first stakeholder of AB Volvo in 2001, which is now running Renault's industrial vehicles activity. A new step of the alliance with Nissan was announced late 2001, including cross shareholding, increase of Renault's stake in Nissan and new joint management structure. Louis Schweitzer is President of the Management Board of Renault-Nissan BV since March 2002.