Download (1393Kb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chief Executive Officer Graham Baker
OVERVIEW OUR BUSINESS OPERATIONAL FINANCIAL RISK GOVERNANCE ACCOUNTS 48 & MARKETPLACE REVIEW REVIEW OUR BOARD OF DIRECTORS ROBERTO QUARTA (67) OLIVIER BOHUON (58) GRAHAM BAKER (48) CHAIRMAN CHIEF EXECUTIVE OFFICER CHIEF FINANCIAL OFFICER Joined the Board in December 2013 and Joined the Board and was appointed Chief -RLQLQJWKH%RDUGDV&KLHI)LQDQFLDO2I²FHULQ appointed Chairman following election by ([HFXWLYH2I²FHULQ$SULO+HUHVLJQHGDV March 2017. shareholders at the April 2014 Annual General a Member of the Nomination & Governance Meeting. He was also appointed Chairman of &RPPLWWHHRQ)HEUXDU\ CAREER AND EXPERIENCE the Nomination & Governance Committee and Graham holds an MA degree in Economics from a Member of the Remuneration Committee on CAREER AND EXPERIENCE Cambridge University and qualified as a Chartered that day. Olivier holds a doctorate in Pharmacy from the Accountant and Chartered Tax Advisor with Arthur University of Paris and an MBA from HEC, Paris. Andersen. In 1995, he joined AstraZeneca PLC where CAREER AND EXPERIENCE He started his career in Morocco with Roussel Uclaf he worked for 20 years, holding multiple senior roles, Roberto is a graduate and a former Trustee of the S.A. and then, with the same company, held a number including Vice President, Finance, International (2013- College of the Holy Cross, Worcester (MA), US. of positions in the Middle East with increasing levels of 2015) with responsibility for all emerging markets, He started his career as a manager trainee at David responsibility. He joined Abbott in Chicago as head of Vice President, Global Financial Services (2011-2013) Gessner Ltd, before moving on to Worcester Controls their anti-infective franchise with Abbott International and Vice President Finance & Chief Financial Officer, Corporation and then BTR plc, where he was a before becoming Pharmaceutical General Manager North America (2008-10). -

Chrysler Affiliate Rewards Program
Chrysler Affiliate Rewards Program If you or your spouse work, or have retired from, one of the companies listed below, you may qualify for pricing as low as 1% Below Factory Invoice on a New FIAT! Check the list of companies below to see if your company qualifies.* A F O AAA-- State of Ohio Members Freightliner Of Tampa, Llc Ocala Freightliner ABB, Inc. Freightliner Of Toledo OCE'- North America Abbott Labs Freightliner Of Utah, Llc Ocean Freightliner, Ltd. Abbott, Nicholson, Quilter, Freightliner Trucks So. Florida Inc. O'connor Gmc, Inc. Esshaki & Youngblood PC Freightliner Twin Ports O'connor Truck Sales, Inc. Abercrombie & Fitch Freightmasters Ohio Machinery Company AboveNet Fresenius Medical dba Ohio CAT Abraxis Bioscience Inc. Fresno Truck Center Oklahoma City Freightliner Accor North America FRIENDLY MOTORCARS Oklahoma Farm Bureau Ace Hardware Corporation Fru-Con Construction Corporation Oklahoma Publishing Company, Action Western Star Fujisawa Healthcare Inc. The (OPUBCO) Actelion Pharmaceuticals US, Inc. Ftl And Ws Of Maine Old Dominion Freight Lines Action Couriers Ftl And Wst Of Tifton Oldcastle Inc. ADVANTAGE Health Solutions, Inc. Ftl Stl Wst Of Odessa Omaha Truck Center Inc Advance Publications Ftl Trucks Of South Florida Omni Care Health Plan Aearo Company Ftl, Stl And Wst Of Montgomery One Call Locators Aetna Ftl,Stl, and Western Star Of Dothan One Source Management Inc Affinia Group Fyda Freightliner Cincinnati Oracle Corporation Agar Truck Sales, Inc. Fyda Freightliner Columbus,Inc Organon Pharmaceuticals USA, Inc. AGCO Corporation Fyda Freightliner Pittsburgh Orlando Freightliner AGFA Corporation Fyda Freightliner Youngstown Orlando Freightliner South Aggreko, LLC ORRIN B HAYES, INC. Agricredit Acceptance LLC G Oscient Pharmaceuticals Agrilink Foods OSI Pharmaceuticals Gabrielli Ford Truck Sales AGSTAR Financial Services Otjen, Van Ert, Stangle, Lieb & Weir, S.C. -

Manufacturers with Signed Rebate Agreements February 1 , 2011
Wisconsin Medicaid Pharmacy Data Table Manufacturers with Signed Rebate Agreements February 1 , 2011 NEWLABELER NAME START END SC NEW LABELER NAME START END SC 00002 ELI LILLY AND COMPANY 1/1/1991 Y 00126 COLGATE ORAL PHARMACEUTICAL 1/1/1991 Y 00003 E R SQUIBB AND SONS INC. 1/1/1991 Y 00131 SCHWARZ PHARMA, INC. 1/1/1991 Y 00004 HOFFMANN LAROCHE INC 1/1/1991 00132 C B FLEET COMPANY INC. 1/1/1991 00005 LEDERLE LABORATORIES 1/1/1991 Y 00135 SMITHKLINE BEECHAM 1/1/1995 Y 00006 MERCK SHARP & DOHME 1/1/1991 Y 00143 WEST-WARD PHARMACEUTICAL C 1/1/1991 Y 00007 SMITHKLINE BEECHAM CORPORATI 1/1/1991 Y 00145 STIEFEL LABORATORIES INC. 1/1/1991 Y 00008 WYETH AYERST LABORATORIES 1/1/1991 Y 00149 PROCTER & GAMBLE PHARMACEU 1/1/1991 Y 00009 PHARMACIA AND UPJOHN 1/1/1991 Y 00168 E FOUGERA AND CO, DIV OF ALTAN 1/1/1991 Y 00013 PHARMACIA AND UPJOHN 1/1/1991 Y 00169 NOVO NORDISK PHARMACEUTICAL 1/1/1991 Y 00015 INVAMED, INC 1/1/1991 Y 00172 ZENITH LABORATORIES, INC 1/1/1991 Y 00023 ALLERGAN INC. 1/1/1991 Y 00173 GLAXOSMITHKLINE 1/1/1991 Y 00024 SANOFI SYNTHELABO 1/1/1991 Y 00178 MISSION PHARMACAL COMPANY 1/1/1991 Y 00025 PHARMACIA CORPORATION 1/1/1991 Y 00182 GOLDLINE LABORATORIES INC 1/1/1991 Y 00026 BAYER CORP PHARMACEUTICAL DI 1/1/1991 Y 00185 EON LABS MANUFACTURING, INC. 1/1/1991 Y 00028 NOVARTIS PHARMACEUTICALS 1/1/1991 Y 00186 ASTRAZENECA LP 1/1/1991 Y 00029 SMITHKLINE BEECHAM CORPORATI 1/1/1991 Y 00187 ICN PHARMACEUTICALS INC. -

ANTARES PHARMA, INC. 100 Princeton South, Suite 300 Ewing, New Jersey 08628 April 27, 2020 Dear Stockholder: You Are Cordially I
ANTARES PHARMA, INC. 100 Princeton South, Suite 300 Ewing, New Jersey 08628 April 27, 2020 Dear Stockholder: You are cordially invited to attend the 2020 Annual Meeting of Stockholders of Antares Pharma, Inc. (the “Company”), to be held at 10:00 a.m., Eastern Time, on Thursday, June 11, 2020 virtually via the Internet at www.virtualshareholdermeeting.com/ATRS2020 (the “Annual Meeting”). For the first time, our annual meeting will be a “virtual meeting” of stockholders, which will be conducted exclusively via the Internet through a virtual web conference. There will not be a physical meeting location, and stockholders will not be able to attend the annual meeting in person. Instructions on how to participate in the Annual Meeting and demonstrate proof of stock ownership are posted at www.virtualshareholdermeeting.com/ATRS2020. This means that you can attend the annual meeting online, vote your shares electronically and submit questions during the online meeting by visiting the above-mentioned website. We believe that hosting a “virtual meeting” will enable greater stockholder attendance and participation from any location around the world. In addition, in response to continued public health concerns related to COVID-19 and group gatherings, we also believe holding a “virtual meeting” will help ensure the health and well-being of our stockholders and other meeting participants. The notice of the 2020 Annual Meeting of Stockholders and the proxy statement that appear on the following pages describe the matters scheduled to come before the meeting. In addition, certain members of our Board of Directors and management team, as well as representatives of KPMG LLP, our independent registered public accounting firm, will be available to answer appropriate questions. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC. -

1999 Annual Report and Form 20-F CONTENTS
1999 Annual Report and Form 20-F CONTENTS Key Achievements in 1999 1 Financial Highlights 2 Shareholder Highlights 5 Chairman’s Statement 6 Chief Executive’s Review 7 Operational Review 8 Safety, Health and Environment 32 In April 1999, Astra AB and Zeneca Group PLC People and Community 34 merged to form AstraZeneca, one of the world’s Financial Review 35 leading pharmaceutical and agrochemical Board of Directors and Officers companies, which provides innovative, effective of the Company 48 products to improve health, nutrition and quality Directors’ Report 49 of life worldwide. The company is research and Financial Statements 55 technology intensive, with extensive international Financial statements and notes development and marketing skills. Its healthcare relating to the financial statements 55 business is strategically focused on seven major Principal subsidiaries, joint ventures therapeutic areas: gastrointestinal, oncology, pain and associates 118 control and anaesthesia, cardiovascular, central Additional information for US investors 120 nervous system, respiratory and infection. Zeneca Group Financial Record 129 Agrochemicals provides crop protection products Shareholder Information 131 designed to improve crop yields and food quality. Exchange rates 138 Definitions 139 Glossary of Terms 140 Cross Reference to Form 20-F IBC Cautionary statement regarding forward-looking statements In order to utilise the ‘Safe Harbor’ provisions of the United States Private Securities Litigation Reform Act of 1995, AstraZeneca is providing the following cautionary statement. This Annual Report and Form 20-F 1999 contains forward-looking statements with respect to the financial condition, results of operations and businesses of AstraZeneca. By their nature, forward-looking statements and forecasts involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. -

Message from the President
DermNewsletter of the American OsteopathicLine College of Dermatology Summer/Fall 2016 Vol. 32, No. 2 Message from the President Greetings from Texas! Before I begin, let us pause and remember the lives of the fallen police officers in grief-stricken Dallas, victims of the tragic event in Nice and the deaths of innocent people around the world. As President of the AOCD, I welcome you to this edition of DermLine. I must extend my special thanks to Dr. Rick Lin, our Immediate Past President, who has supported me tirelessly during this term. His friendship and his willingness to share his knowledge have proven invaluable. I also would like to acknowledge the Board of Trustees, Marsha Wise and the entire AOCD team – your support of me has been tireless and humbling. While writing this brief missive, I am preparing for a trip to Chicago to attend the annual AOA House of Delegates meeting. I am reminded of the great progress we have made in moving the AOCD forward! A single accreditation system for graduate medical education programs in the United States marks a true milestone in the lives of graduates of osteopathic institutions. Our doctors will now complete their residency and/or fellowship education in ACGME-accredited programs and demonstrate achievement of common milestones and competencies without impediments. It is my hope that osteopathic and allopathic graduates who train together, side by side, will no longer be a divided group. Our work is not done! As I mentioned in my last message, the AOCD remains a strong organization. I am proud of our heritage and mindful of the work that lies ahead. -

Hospitals, Big Pharma, Hmos & the Health Care War Economy
The Second Annual IHSP Hospital 200: Hospitals, Big Pharma, HMOs & the Health Care War Economy Version 1.1, Embargoed Until September 8, 2004 The Institute for Health & Socio-Economic Policy (IHSP) is a non-profit policy and research group. The IHSP focus is current political/economic policy analysis in health care and other industries and the constructive engagement of alternative policies with international, national, state and local bodies to enhance promote and defend the quality of life for all. The IHSP has a number of Advisory Boards comprised of analysts and policy activists with expertise in their respective industries of interest. Advisory Boards include Health Care, Alternative Technologies, Energy, Communications, etc. The Health Care Advisory Board is comprised of scholars from the Albert Einstein College of Medicine, Boston University, Harvard University, the Canadian National Federation of Nurses’ Unions, the New School in New York and the University of California. Institute for Health & Socio-Economic Policy 21 C Orinda Way #361 Orinda CA 94563 Tel: 510-267-0634 Fax: 510-267-0635 e-mail: [email protected] I. ABOUT THE INSTITUTE FOR HEALTH & SOCIO-ECONOMIC POLICY ............................ 4 II. PRINCIPAL FINDINGS.................................................................................................................. 5 III. DATA SOURCES.............................................................................................................................. 8 A. METHODOLOGY EMPLOYED IN THIS REPORT.................................................................................. -

Astrazeneca Notice of AGM 2007
NOTICE OF ANNUALAND SUMMARY GENERAL MEETINGFINANCIAL STATEMENT 2007 AND SHAREHOLDERS’ cIRCULAR WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd LETTER FROM THE CHAIRMAN 1 LETTER FROM THE CHAIRMAN THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE AttENTION. IF YOU ARE or incurring because of the Political Parties, IN ANY DOUBT ABOUT ITS CONTENTS OR WHAT ACTION YOU SHOULD TAKE, YOU SHOULD Elections and Referendums Act 2000 in the CONSULT YOUR INDEPENDENT FINANCIAL ADVISER. IF YOU HAVE SOLD OR TRANSFERRED ALL UK (“the Act”). OF YOUR ASTRAZENECA ORDINARY SHARES YOU SHOULD SEND THIS DOCUMENT AND THE ACCOMPANYING DOCUMENTS TO THE PURCHASER OR TRANSFEREE OR TO THE STOCKBROKER, The Company has no intention of changing its BANK OR OTHER AGENT THROUGH WHOM THE SALE OR TRANSFER WAS EFFECTED FOR current practice of not making donations to TRANSMISSION TO THE PURCHASER OR TRANSFEREE. political parties in the EU and it will not do so without the specific endorsement of its shareholders. However, the Act defines DEAR SHAREHOLDER Senior Independent Director. Sir Peter was ‘political organisation’ widely so as to include, On behalf of the Board of AstraZeneca PLC, first appointed to the Board in January 1995. amongst other things, an organisation that I enclose various documents concerning your carries on activities that are capable of being shareholding in the Company. These are: On behalf of the whole Board, I would like reasonably regarded as intended to influence to express our gratitude to Erna and Sir public support for a political party in any EU 1 A Shareholders’ Circular incorporating Peter for their considerable contribution to member state or to influence voters in relation the formal Notice of the Annual General AstraZeneca’s success and wish them both to any referendum in any EU member state. -
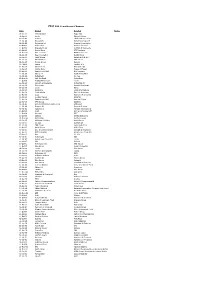
FTSE 100 Constituent History Updated
FTSE 100 Constituent Changes Date Added Deleted Notes 19-Jan-84 CJ Rothschild Eagle Star 02-Apr-84 Lonrho Magnet Sthrns. 02-Jul-84 Reuters Edinburgh Inv. Trust 02-Jul-84 Woolworths Barrat Development 19-Jul-84 Enterprise Oil Bowater Corporation 01-Oct-84 Willis Faber Wimpey (George) 01-Oct-84 Granada Group Scottish & Newcastle 01-Oct-84 Dowty Group MFI Furniture 04-Dec-84 Brit. Telecom Matthey Johnson 02-Jan-85 Dee Corporation Dowty Group 02-Jan-85 Argyll Group Berisford (S.& W.) 02-Jan-85 MFI Furniture RMC Group 02-Jan-85 Dixons Group Dalgety 01-Feb-85 Jaguar Hambro Life 01-Apr-85 Guinness (A) Enterprise Oil 01-Apr-85 Smiths Inds. House of Fraser 01-Apr-85 Ranks Hovis McD. MFI Furniture 01-Jul-85 Abbey Life Ranks Hovis McD. 01-Jul-85 Debenhams I.C. Gas 06-Aug-85 Bnk. Scotland Debenhams 01-Oct-85 Habitat Mothercare Lonrho 02-Jan-86 Scottish & Newcastle Rothschild (J) 08-Jan-86 Storehouse Habitat Mothercare 08-Jan-86 Lonrho B.H.S. 01-Apr-86 Wellcome EXCO International 01-Apr-86 Coats Viyella Sun Life Assurance 01-Apr-86 Lucas Harrisons & Crosfield 01-Apr-86 Cookson Group Ultramar 21-Apr-86 Ranks Hovis McD. Imperial Group 22-Apr-86 RMC Group Distillers 01-Jul-86 British Printing & Comms. Corp Abbey Life 01-Jul-86 Burmah Oil Bank of Scotland 01-Jul-86 Saatchi & S. Ferranti International 01-Oct-86 Bunzl Brit. & Commonwealth 01-Oct-86 Amstrad BICC 01-Oct-86 Unigate Smiths Industries 09-Dec-86 British Gas Northern Foods 02-Jan-87 Hillsdown Holdings Argyll Group 02-Jan-87 I.C. -

[ Emc-Lusinnufll'lergcrta'rgets
US 20070255633Al (19) United States (12) Patent Application Publication (10) Pub. N0.: US 2007/0255633 A1 Kridel (43) Pub. Date: NOV. 1, 2007 (54) SYSTEMS AND METHODS FOR INVESTING (52) US. Cl. ....................................................... .. 705/35 (76) Inventor: FIVJigiam J. Kridel, New York, NY (57) ABSTRACT The present invention discloses systems and methods for Correspondence Address? creating and managing ?nancial instruments and indexes PAUL’ HASTINGS’ JANOFSKY & WALKER comprised of securities for companies in subsectors of the LLP economy. These ?nancial instruments alloW investment in R0' BOX 919092 subsectors of the economy Will still being able to minimize SAN DIEGO CA 92191-9092 ’ risk by diversi?cation. The indexes serve as benchmarks for (21) App1_ NO; 11/465,768 companies in the subsectors of the economy. A procedure may be used to identify the securities to include in the (22) Filed: Allg- 18, 2006 ?nancial instruments. This procedure may include (a) iden _ _ tifying securities for companies in a sector of the economy; Related U's‘ Apphcatlon Data (b) limiting the identi?ed securities to those for companies (60) Provisional application No. 60/778,492, ?led on Mar. in a SubSeCIOr Of the SBCIOI‘ Of the economy; (0) applying 1, 2006. focus rules to further limit the identi?ed securities to those _ _ _ _ for companies Who are focused in the subsector of the Pubhcatlon Classl?catlon economy; and (d) limiting the securities included in the (51) Int, Cl, ?nancial instrument or index to those that satisfy other G06Q 40/00 (2006.01) objective criteria. ETF RULE. SET->| APPLICATION’ ' "1 _. -

2015 Year-End Fda and Health Care Compliance and Enforcement Update – Drugs and Devices
January 19, 2016 2015 YEAR-END FDA AND HEALTH CARE COMPLIANCE AND ENFORCEMENT UPDATE – DRUGS AND DEVICES To Our Clients and Friends: While massive mergers and pharmaceutical pricing dominated industry headlines in 2015, the U.S. Government continued to shape the drug and device industries through enforcement actions and other regulatory activities this past year. The U.S. Department of Justice ("DOJ") and the U.S. Food and Drug Administration ("FDA"), in particular, pursued drug and device companies--and, in some instances, individual executives at those companies--for a variety of alleged misconduct. Meanwhile, FDA continued to push current priority goals such as developing more efficient pathways to market for many drugs and devices and promoting greater cybersecurity protections for medical devices. But the second half of 2015 also saw important developments pushing back on FDA's regulatory authority; another federal court invalidated FDA's efforts to police truthful off-label promotion, and congressional and industry stakeholders explored alternative schemes for oversight of laboratory developed tests ("LDTs"). Amidst all of this action, the past year's legislative stagnation was especially evident--with one notable exception. We reported in our 2015 Mid-Year FDA and Health Care Compliance and Enforcement Update on Drugs and Devices ("2015 Mid-Year Update") that the U.S. House of Representatives passed the 21st Century Cures Act, but the last six months saw little (if any) legislative progress. In the meantime, federal and state legislators toyed with several proposals designed to increase government involvement in drug prices. Below, we discuss the past six months' developments in the regulation of both drugs and devices.