Emergency Responder Radioactive Material Quick Reference Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The International Commission on Radiological Protection: Historical Overview
Topical report The International Commission on Radiological Protection: Historical overview The ICRP is revising its basic recommendations by Dr H. Smith Within a few weeks of Roentgen's discovery of gamma rays; 1.5 roentgen per working week for radia- X-rays, the potential of the technique for diagnosing tion, affecting only superficial tissues; and 0.03 roentgen fractures became apparent, but acute adverse effects per working week for neutrons. (such as hair loss, erythema, and dermatitis) made hospital personnel aware of the need to avoid over- Recommendations in the 1950s exposure. Similar undesirable acute effects were By then, it was accepted that the roentgen was reported shortly after the discovery of radium and its inappropriate as a measure of exposure. In 1953, the medical applications. Notwithstanding these observa- ICRU recommended that limits of exposure should be tions, protection of staff exposed to X-rays and gamma based on consideration of the energy absorbed in tissues rays from radium was poorly co-ordinated. and introduced the rad (radiation absorbed dose) as a The British X-ray and Radium Protection Committee unit of absorbed dose (that is, energy imparted by radia- and the American Roentgen Ray Society proposed tion to a unit mass of tissue). In 1954, the ICRP general radiation protection recommendations in the introduced the rem (roentgen equivalent man) as a unit early 1920s. In 1925, at the First International Congress of absorbed dose weighted for the way different types of of Radiology, the need for quantifying exposure was radiation distribute energy in tissue (called the dose recognized. As a result, in 1928 the roentgen was equivalent in 1966). -

ACUTE RADIATION SYNDROME: Diagnosis and Treatment
ACUTE RADIATION SYNDROME: Diagnosis and Treatment Badria Al Hatali, MD Medical Toxicologist Department of Environmental and Occupational Health MOH - Oman Objectives Provide a review of radiation basics and acute radiation sickness Discuss diagnostic tools and triage tools for Acute Radiation Syndromes Discuss management of Acute Radiation Syndromes Energy traveling over a distance as Waves Particles • Gamma rays • Alpha • X-rays • Beta • Radio waves • neurons Non-ionizing vs Ionizing Radiation • High energy • Low energy • Removes orbital electrons • Does not remove orbital from atoms > DNA electrons from atom damage Radioactive Decay Process to Remove excess energy from atomic nuclei Nuclei emit rays or particles to decrease nuclear energy Radioactive materials have unstable nuclei with excess energy Ionizing Radiation Dose • Radiation absorb dose (RAD): the amount of energy absorbed by the body. 1 cGy = 0.01 J/kg (USA) • Gray (Gy): expressed as absorbed energy per unit mass of tissue. 100 rad =100 cGy =1 J/kg (SI) • Roentgen Equivalent Man (REM) relates the absorbed dose in human tissue to the effective biological damage of the radiation (USA) • Sievert (Sv): the absorbed dose in human tissue to the effective biological damage of the radiation (SI) Radioactivity Biological And Effective Half-lives Biological half-life is the time to remove half of radioactive element from body Effective half-life is the combined effect of radioactive decay & biological elimination Effective half-life is always shorter than either physical or biological half-lives Biological Effects of Ionizing Radiation Direct damage Chromosome Other biochemical E.g. alpha and beta particles Indirect damage Chemical changes due to radiolysis of water in cell E.g. -

Ionizing Radiation in Earth's Atmosphere and in Space Near Earth May 2011 6
Federal Aviation Administration DOT/FAA/AM-11/9 Office of Aerospace Medicine Washington, DC 20591 Ionizing Radiation in Earth’s Atmosphere and in Space Near Earth Wallace Friedberg Kyle Copeland Civil Aerospace Medical Institute Federal Aviation Administration Oklahoma City, OK 73125 May 2011 Final Report OK-11-0024-JAH NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. ___________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: www.faa.gov/library/reports/medical/oamtechreports Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-11/9 4. Title and Subtitle 5. Report Date Ionizing Radiation in Earth's Atmosphere and in Space Near Earth May 2011 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Friedberg W, Copeland K 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) FAA Civil Aerospace Medical Institute P.O. Box 25082 11. Contract or Grant No. Oklahoma City, OK 73125 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. Washington, DC 20591 14. Sponsoring Agency Code 15. Supplemental Notes 16. Abstract The Civil Aerospace Medical Institute of the FAA is charged with identifying health hazards in air travel and in commercial human space travel. -

Radiation Biology: Cell Biology
Agenda Radiologic Units: • Greys, Sieverts, Coulombs per kg, & What You Need to Becquerel's Know • Conventional Units TODD VAN AUKEN M.ED. RT (R)(MR) • Other Concepts (LET, Q-Factor, Effective Dose, NCRP Report # 116) • Crazy Happenings with Radiation Coulomb per kg or C/kg-Exposure (X) Erythema Dose • A Coulomb (C) is the basic unit of electric charge • Measurement of positive and negative particles created when radiation ionizes atoms in dry air (Radiation intensity in the air) • Unit of Exposure for X-rays and Gamma Rays (Restricted to Photons only) • Used for x-ray equipment calibration (X-ray tube output) • Conventional Unit: Roentgen (R) Air Kerma Gray (Gy)-Absorbed Dose (D) • Kinetic energy is transferred from the primary beam • Amount of energy per unit mass absorbed by an (photons) to the patient (e-) irradiated object • SI quantity that can be used to measure radiation • 1 joule (J) of energy absorbed concentration transferred to a point at the surface of a in 1 g of absorbing material patient’s or radiographer’s body • Absorption of energy may • The energy transfer is called the kinetic energy result in biological damage released in the material or kerma (air kerma) • As the Z# of the object • Dose Area Product (DAP)- sum total of Air Kerma increases the absorbed dose over the exposed area of the patient’s surface (entire increases amount of energy delivered to the patient) • Conventional Unit: RAD (Radiation Absorbed Dose) Linear Energy Transfer Linear Energy Transfer Low LET Low LET High LET High LET LET= Rate at which energy -

Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements?
www.JICReadiness.com Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements? A: Since nuclear radiation affects people, we must be able to measure its presence. We also need to relate the amount of radiation received by the body to its physiological effects. Two terms used to relate the amount of radiation received by the body are exposure and dose. When you are exposed to radiation, your body absorbs a dose of radiation. As in most measurement quantities, certain units are used to properly express the measurement. For radiation measurements they are... Roentgen: The roentgen measures the energy produced by gamma radiation in a cubic centimeter of air. It is usually abbreviated with the capital letter "R". A milliroentgen, or "mR", is equal to one one-thousandth of a roentgen. An exposure of 50 roentgens would be written "50 R". Rad: Or, Radiation Absorbed Dose recognizes that different materials that receive the same exposure may not absorb the same amount of energy. A rad measures the amount of radiation energy transferred to some mass of material, typically humans. One roentgen of gamma radiation exposure results in about one rad of absorbed dose. Rem: Or, Roentgen Equivalent Man is a unit that relates the dose of any radiation to the biological effect of that dose. To relate the absorbed dose of specific types of radiation to their biological effect, a "quality factor" must be multiplied by the dose in rad, which then shows the dose in rems. For gamma rays and beta particles, 1 rad of exposure results in 1 rem of dose. -

Note on Using Low-Sensitivity Geigers with The
Canadian Nuclear Society Working with less sensitive Geiger-Müeller Instruments www.cns-snc.ca Education Teachers … Page 1 of 4 Introduction The CNS Ionising Radiation Workshop features the use of higher sensitivity Geiger-Müeller detectors than most high school science teachers have access to. This note illustrates the impact of using lower sensitivity instruments with weak sources – AND shows how they may be used with the more intense sources described in the Workshop Notes. Damn lies and statistics The collection of count data with an instrument such as a Geiger detector is made using assumptions that are important to the analysis of the data set. These include: 1. Stable background radiation level 2. Consistent geometry 3. Stable sensitivity Lower sensitivity detectors have correspondingly lower average count rates when monitoring an ionising radiation source, including background radiation. Consequently it is difficult to detect the presence of ionising radiation sources that provide only a modest increase over the background rate. This limitation is due to the statistics of the count data. The pulses from a Geiger counter are collected as a number of counts in a time interval, for example the number of counts in a one minute interval. These are integer values. The average number of counts over a number of one-minute counting intervals is a real number. The one-minute interval data set may be described in terms of a Poisson probability distribution. For a data set with a large number of sample intervals and a sufficiently high average number per interval the probability distribution tends toward a Normal (or Gaussian) distribution. -

Sludge Stabil: Hanford Site, Zation at the Plutonium Finish
DOE~A-0978 . Sludge Stabil:zation at the Plutonium Finish: ng Plant, -, Hanford Site, Mchland, Washington U.S. Dep-ent of Energ NcMand, Wash@on October 1994 _—_— DOE~A-0978 I I ( I —....— .—.. U,S. Deparment of Energy Glossary Glossary Acronp md Abbreviation ARF airborne release fraction ASIL Acceptable Source Impact Level D&D dmontamination and decommissioning DOE U.S. Department of Energy EA Environment Assessment Ecology State of Washington Department of Ecology EDE effective dose equivalent EIS Environment Impact Statement EPA U.S. Environment Protection Agency FFTF Fmt Flux Test Facflity HEPA High-Efficiency Partictiate Air LFL lower flammability limit LCF latent cancer fatrdity NESHAP Natioti Emission S~dards for H=dous Air Pollutants PFP Plutonium Finishing Plant PRF Plutonium Reclamation Factiity PUREX Plutonium-Uranium Extraction RCW Resource Conservationand Recove~ A~ of 1976 rem Roentgen Equivalent Man RMC ~ remote mechanical “C” TBP tributyl phosphate TLV-STEL Threshold Limit Value - Short Term Exposure Limit TLV-TWA Threshold Limit Value - Time Weighted Average Tri-Party Agreement Hanford Federal Facilip Agreement and Consent Order TRUS@ Transuranic Waste Storage and Assay Factiity WAC WmhingtonAdministr~.ve Code WIPP Waste Isolation Ptiot Plant Definition of Selected Tem Effective Dose Eauivdent. A value used for estimating the toti risk of potential health effects from radiation exposure. This estimate is the sum of the committed effective dose equivalent from interti deposition of radionuclidm in the body and the effective dose equivalent from exterti radiation received during a year. Latent cancer fatalitv: The excess mcer fatiities h a population due to exposure to a carcinogen. DOEEA-0978 October 1994 — -—— — U.S. -

Hanford Fire Department Radiation Fundamentals for Emergency Responders
Objectives – To understand the hazards of responding to events involving radioactive materials – To know the fundamentals of radioactive contamination – To understand the biological affects of exposure to radioactive materials – To know how to respond to hazmat events involving radioactive materials RADIOLOGICAL TERMS • CURIE (Ci) – The basic unit of activity. A quantity of any radionuclide that undergoes an average of 37 billion transformations per second. – One curie is the approximate activity of 1 gram of radium. – Named after Marie and Pierre Curie, who discovered radium in 1898 • Rad (radiation absorbed dose)- – Measures a quantity called “absorbed dose” which means the amount of energy actually absorbed in a material. – The rad measures any type of radiation, but it does not describe the biological effects. • Rem (roentgen equivalent man)- – Measures a quantity called “equivalent dose” which relates the absorbed dose in human tissue to the resulting biological damage. – This measurement is necessary because not all radiation has the same biological effect. – The rem measurement is obtained by measuring the rad and multiplying it by a quality factor that is unique to a specific type of radiation. • Roentgen (R)- – A unit of exposure to ionizing radiation. – It is the amount of gamma or x-rays required producing ions carrying 1 electrostatic unit of electrical charge in 1 cubic centimeter of dry air under standard conditions. – Named after Wilhelm Roentgen, German scientist who discovered x-rays in 1895. SYSTEME INTERNATIONAL Traditional -

Useful Information
USEFUL INFORMATION Radiation and Radioactivity Radioactivity is a characteristic of some elements that have unstable atomic nuclei, which spontaneously disintegrate or “decay” into atomic nuclei of another isotope or element. The nuclei decay until only a stable, nonradioactive isotope remains. Depending on the isotope, this process can take anywhere from less than a second to billions of years. Atoms that emit radiation are called radionuclides. Radionuclides are unstable isotopes of an element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses. For example, the element hydrogen has two stable isotopes, hydrogen•1 ( 1H) and hydrogen•2 ( 2H) (deuterium), and one radioactive isotope, hydrogen•3 (3H) (tritium). The superscript preceding the element’s symbol identifies the atomic mass, which is the number of protons plus neutrons in the nucleus. Thus, 1H has one proton and no neutrons, 2H has one proton and one neutron, and3H has one proton and two neutrons. When radioactive atoms decay by emitting radiation, the daughter products that result may be either radioactive or stable. Generally, radionuclides with high atomic numbers, such as uranium•238 and plutonium•239, have many generations of radioactive progeny. For example, the radioactive decay of plutonium•239 creates uranium•235, thorium•231, protactinium•231, and so on, through 11 progeny until only the stable isotope lead•207 remains. Radionuclides with lower atomic numbers often have no more than one daughter. For example, strontium•90 has one radioactive daughter, yttrium•90, which finally decays into stable zirconium; cobalt•60 decays directly to stable nickel with no intermediate nuclide. -
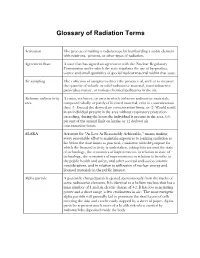
Glossary of Radiation Terms
Glossary of Radiation Terms Activation The process of making a radioisotope by bombarding a stable element with neutrons, protons, or other types of radiation. Agreement State A state that has signed an agreement with the Nuclear Regulatory Commission under which the state regulates the use of by-product, source and small quantities of special nuclear material within that state. Air sampling The collection of samples to detect the presence of, and/or to measure the quantity of volatile or solid radioactive material, non-radioactive particulate matter, or various chemical pollutants in the air. Airborne radioactivity A room, enclosure, or area in which airborne radioactive materials, area composed wholly or partly of licensed material, exist in concentrations that: (1) Exceed the derived air concentration limits, or (2) Would result in an individual present in the area without respiratory protection exceeding, during the hours the individual is present in the area, 0.6 percent of the annual limit on intake or 12 derived air concentration-hours. ALARA Acronym for "As Low As Reasonably Achievable," means making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken, taking into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, and other societal and socioeconomic considerations, and in relation to utilization of nuclear energy and licensed materials in the public interest. Alpha particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. -

GLOSSARY of BASIC RADIATION PROTECTION TERMINOLOGY ABSORBED DOSE: the Amount of Energy Absorbed, As a Result of Radiation Passin
GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY ABSORBED DOSE: The amount of energy absorbed, as a result of radiation passing through a material, per unit mass of material. Measured in rads (1 rad = 100 ergs/gm) or grays, the SI unit, where 1 gray = 100 rads. ABSORPTION: Process by which energy from radiation is transferred to matter by interactions with the constituents of the matter. A result is a reduction in the intensity of the radiation. ACTIVITY: The strength of a radioactive source, i.e. the number of radioactive atoms decaying per unit of time. (See Radioactivity) Measured in Curies or Bequerels, where 1 Curie = 3.7 x 1010 Bequerels. ALPHA PARTICLE: A particle which is similar to the helium nucleus, consisting of two protons and two neutrons, but is stripped of its orbital electrons. It has a charge of +2 and is the least penetrating of the three common types of radiation. Usually a hazard only occurs when an alpha-emitting substance has entered the body by inhalation, ingestion or absorption through the skin. ANNIHILATION RADIATION: Photons produced when a particle and its anti-particle interact and annihilate each other. The photons have an energy of 0.511 MeV each and are emitted in opposite (180°) directions. ATOMIC NUMBER: The number of protons in the nucleus of an atom. ATOMIC WEIGHT: Number approximately equal to the total number of protons and neutrons in the nucleus of an atom, i.e. the mass of an atom. ATTENUATION: A reduction in the intensity of radiation as it passes through matter. AVALANCHE: The buildup of ionization by electrons produced in a G-M tube by the primary ionization as the electrons drift toward the collector. -

Table of Contents
Topic No. 675-050-001 Radiation Safety Manual Revision: May 13, 2003 Radiation Effective: June 1, 2000 TABLE OF CONTENTS CHAPTER 7 - RADIATION 7.1 PURPOSE ........................................................................................................ 1 7.2 SCOPE ............................................................................................................. 1 7.3 BASIC CONCEPTS OF NUCLEAR SCIENCE ................................................. 1 7.3.1 About Atoms .............................................................................................. 1 7.3.2 Elements.................................................................................................... 1 7.3.3 Isotopes ..................................................................................................... 2 7.3.4 Half-life....................................................................................................... 2 7.3.5 Radioactivity .............................................................................................. 2 7.4 TYPES OF RADIATION.................................................................................... 2 7.4.1 Ionizing Radiation ...................................................................................... 2 7.4.2 Non-Ionizing Radiation............................................................................... 3 7.5 MEASURES OF RADIATION ........................................................................... 3 7.5.1 Becqerel (Bq)............................................................................................