Michigan Medicine Site Profile June 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UH-CVC-Guidebook.Pdf
Table of Contents Welcome ..................................... 4 What is Patient and Family Centered Care? . 5 Maps ......................................... 6 Communicating With Your Health Care Team ....... 7 What’s a Teaching Hospital? . 7 A Who’s Who of Doctors and Nurses . 8 Therapists and Other Caregivers . 9 Patient Safety — Our First Priority ............... 10 What to Ask Your Doctors and Other Health Care Team Members . 11 Hand Hygiene ................................. 12 Infection Control .............................. 13 The Importance of Staying Clean . 13 What to Expect: The Hospital Routine ............ 14 Daily Routine . 14 Medical Rounds . 14 Nursing Care . 14 Your Room ................................... 15 Call Button . 15 White Board . 15 Television . .. 15 Dining Services ............................... 16 Inpatient Tray Delivery ......................... 16 Guest Trays . 16 Nourishment Rooms . 16 Retail Food Dining/Coffee Shops . 16 Patient Visitor Accommodations . 19 Med Inn . 19 Michigan Transplant House . 19 Quiet Hours . 19 2 University Hospital and Frankel Cardiovascular Center Adult Guidebook Parking ...................................... 20 Patient Relations and Clinical Risk ............... 34 Parking Rates . 20 Going Home .................................. 37 Outpatient Day Parking Pass . 21 Care Management . 37 Inpatient Visitor Parking Pass . 21 Outpatient Pharmacy . 38 Valet Services . 21 University of Michigan Home Care Services . 39 Support Care Team Members ................... 22 Opportunities After -

Plant Extension Report
THE UNIVERSITY OF MICHIGAN REGENTS COMMUNICATION ITEM FOR INFORMATION Subject: Plant Extension Background and Summary: The majority of projects in planning have been paused to reduce expense in design and subsequent construction. These projects will be re-evaluated along with the university's financial condition. Construction activities on University of Michigan projects continued as shown on the attached schedules during the months of December 2020 and January 2021. The projects listed below have been completed, except for specific items, and will no longer be included in this report. • Alumni Center Renovation • Flint William R. Murchie Science Building Expansion • Ford Motor Company Robotics Building • Edward Henry Kraus Building Renovation and Addition • Ann and Robert H. Lurie Biomedical Engineering Building First Floor Renovations • Michigan Medicine Eisenhower Corporate Park West HomeMed Pharmacy Clean Room Renovations • Michigan Medicine A. Alfred Taubman Health Care Center Air Handling Equipment Replacement • South University Pavement and Utility Improvements • Wall Street West Parking Structure Also attached is the quarterly report on construction activities between $500,000 and $3,000,000 that were financially completed during the period of October 1 through December 31, 2020. Respectfully submitted, ________________________ Kevin P. Hegarty Executive Vice President and Chief Financial Officer February 2021 Attachment PROJECTS IN PLANNING Status as of January 22, 2021 February 2021 Proposed Project Project Budget Source of -
2018-02-III-1.Pdf
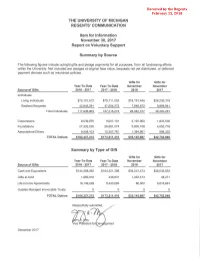
THE UNIVERSITY OF MICHIGAN REGENTS' COMMUNICATION Item for Information November 30, 2017 Report on Voluntary Support Summary by Source The following figures include outright gifts and pledge payments for all purposes, from all fundraising efforts within the University. Not included are pledges at original face value, bequests not yet distributed, or deferred payment devices such as insurance policies. Gifts for Gifts for Year-To-Date Year-To-Date November November Source of Gifts 2016- 2017 2017-2018 2016 2017 Individuals Living Individuals $75,101,612 $79,711,002 $18,191,445 $26,230;319 Realized Bequests 42,845,291 47,504,272 7,848,872 9,989,941 Total Individuals 117,946,903 127,215,274 26,040,317 36,220,261 Corporations 9,539,975 9,057,101 2,120,900 1,833,535 Foundations 27,332,230 26,581,274 5,600,108 4,050,718 Associations/Others 9,508,103 10,357,761 1,384,661 598,332 TOTAL Dollars $164,327,212 $173,211,410 $35,145,987 $42,702,846 Summary by Type of Gift Gifts for Gifts for Year-To-Date Year-To-Date November November Source of Gifts 2016- 2017 2017- 2018 2016 2017 Cash and Equivalents $144,288,065 $164,321,395 $34,012,4 73 $36,035,932 Gifts-In-Kind 1,889,918 446,931 1,053,513 48,271 Life Income Agreements 18,149,228 8,443,083 80,000 6,618,643 Outside Managed Irrevocable Trusts 0 0 0 0 TOTAL Dollars $16413271212 $17312111410 $3511451987 $4217021846 December 2017 Item for Information November 30, 2017 Report on Voluntary Support Summary by Source State of Michigan Outside of State State of Michigan Outside of State Year to Date Year -

Michigan Medicine Department of Pharmacy
Michigan Medicine Department of Pharmacy Patient Care · Education · Research · Community 2016-2017 Annual Report 2 Table of Contents Introduction—————————————————————————–————————–4-5 University Hospital/ Cardiovascular Center Pharmacy—————–———–———————6 Medication Use Systems—————————————————————————————7 Children & Women’s Center Pharmacy—————————————–———–—————8-9 Outpatient and Ambulatory Care Pharmacy————————————–———————–10-13 Specialty Pharmacy———————————————————–———–————————14-15 Infusion Pharmacy————————————————————————–———–———16-17 Research Pharmacy————————————————————————–——————18-19 Medication Use Policy————————————————————–——————–——–20 Antibiotic Stewardship————————————————————————–—————21 HomeMed Pharmacy & Specialty Infusion————————————————–————22-23 Medication Use Technology————————————————————––——————24 Medication Use Information———————————————————–———————–25 Pharmacy Residents—————————————–—————————–———————–26 Internship Opportunity ———————————————————–—————————–27 2 2 3 Our Leadership Team Jake Holler Director — Acute Care Pharmacy Services Stan Kent Chief Pharmacy Officer Bruce Chaffee, John Clark Mike Kraft Associate Chief Pharmacy Officer Assistant Director — Assistant Director — Quality, Safety, Regulatory Education and Research Lindsey Kelley Rachel Cortis Director — Ambulatory Pharmacy Services Assistant Director — Business Services 3 3 3 4 Annual Report Introduction I am very pleased to present this 2016-17 annual report highlighting just a few of the many accomplishments of the Univer- sity of Michigan -
THE UNIVERSITY of MICHIGAN REGENTS' COMMUNICATION Item
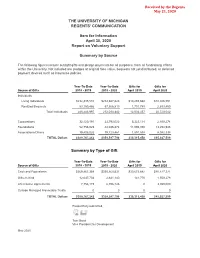
THE UNIVERSITY OF MICHIGAN REGENTS' COMMUNICATION Item for Information April 30, 2020 Report on Voluntary Support Summary by Source The following figures include outright gifts and pledge payments for all purposes, from all fundraising efforts within the University. Not included are pledges at original face value, bequests not yet distributed, or deferred payment devices such as insurance policies. Year-To-Date Year-To-Date Gifts for Gifts for Source of Gifts 2018 - 2019 2019 - 2020 April 2019 April 2020 Individuals Living Individuals $232,335,510 $214,667,623 $10,293,668 $18,026,591 Realized Bequests 53,150,486 57,366,819 1,710,789 2,693,450 Total Individuals 285,485,997 272,034,442 12,004,457 20,720,042 Corporations 32,120,191 23,793,022 5,323,114 2,930,274 Foundations 52,756,029 44,046,874 11,096,050 13,294,945 Associations/Others 19,405,025 19,723,461 1,891,838 8,082,338 TOTAL Dollars $389,767,242 $359,597,798 $30,315,458 $45,027,599 Summary by Type of Gift Year-To-Date Year-To-Date Gifts for Gifts for Source of Gifts 2018 - 2019 2019 - 2020 April 2019 April 2020 Cash and Equivalents $369,961,359 $350,160,531 $30,673,682 $40,427,321 Gifts-In-Kind 12,047,703 2,641,140 141,776 1,550,278 Life Income Agreements 7,758,179 6,796,126 0 3,050,000 Outside Managed Irrevocable Trusts 0 0 0 0 TOTAL Dollars $389,767,242 $359,597,798 $30,315,458 $45,027,599 Respectfully submitted, Tom Baird Vice President for Development May 2020 Item for Information April 30, 2020 Report on Voluntary Support Summary by Source State of Michigan Outside of State -

Interim Policy on Sexual and Gender-Based Misconduct
THE UNIVERSITY OF MICHIGAN INTERIM POLICY ON SEXUAL AND GENDER-BASED MISCONDUCT Effective August 14, 2020 The University of Michigan Interim Policy On Sexual and Gender-Based Misconduct Table of Contents Effective August 14, 2020 I. POLICY STATEMENT 1 II. POLICY DEFINITIONS 2 III. POLICY SCOPE AND APPLICABILITY 5 IV. APPLICABLE PROCEDURES UNDER THIS POLICY 6 V. CONFIDENTIAL AND NON-CONFIDENTIAL RESOURCES 6 A. Confidential Resources ........................................................................................... 7 B. Non-Confidential Resources ................................................................................... 9 VI. REPORTING 10 A. Reporting to the University ................................................................................... 10 1. Contact the Office for Institutional Equity and Title IX Coordinator ...... 10 2. Reports to Individuals with Reporting Obligations .................................. 11 3. Anonymous Reporting .............................................................................. 12 B. Reporting to Law Enforcement ............................................................................. 13 C. Receipt by the University of Reports of Prohibited Conduct ............................... 14 D. Additional Information about Reporting .............................................................. 14 1. Time Frame for Reporting an Incident to the University ......................... 14 2. Information on Amnesty to Students When Reporting Prohibited Conduct to the University ....................................................................................... -

The Science, Practice, and Teaching of Medicine at the University of Michigan, 1850-1941
Not Just Any Medical School: The Science, Practice, And Teaching Of Medicine At The University Of Michigan, 1850-1941 Horace Willard Davenport Public Health Chronicles - National Center for Biotechnology. Not Just Any Medical School: The Science, Practice, and Teaching of Medicine at the University of Michigan, 1850-1941 Horace W. Davenport on Amazon.com. History University of Michigan Medical School The Early History Medicine at Michigan Not Just Any Medical School: The Science, Practice, and Teaching. Title, Not just any medical school: the science, practice, and teaching of medicine at the University of Michigan, 1850-1941 / Horace W. Davenport. Pub Info, Ann Washington Post editor recounts personal journey to unmask family. Not Just Any Medical School: The Science, Practice and Teaching of Medicine at the University of Michigan, 1850-1941 by Horace W. Davenport, Dr. Echols' list of Google books for Civil War - Medical Antiques Still, the early faculty at our fledgling medical school were men of amazing vision. Ph.D., noted in his book Not Just Any Medical School: The Science, Practice and Teaching of Medicine at the University of Michigan, 1850-1941, By the turn Not Just Any Medical School: The Science, Practice, and Teaching of. Author: Horace W. Davenport, Title: Not Just Any Medical School: The Science, Practice, and Teaching of Medicine at the University of Michigan, 1850-1941 Access article in PDF. Book Review. Not Just Any Medical School: The Science, Practice, and Teaching of Medicine at the University of Michigan, 1850-1941 Lakeland Library Cooperative /All Locations Not Just Any Medical School: The Science, Practice, and Teaching of Medicine at the University of Michigan, 1850-1941. -

Fall 2020 the Legacy of a 2017 Alumnus Who Represented ‘Everything the School Stands For.’
INSIDE: A SAINTLY EDUCATOR Notre Dame’s Fr. Leon discusses 22 the founder of the Society of Mary and his road to sainthood. IRISH A QUIET GRACE 30 A new scholarship offered to ISSUE 25 Notre Dame students carries Fall 2020 the legacy of a 2017 alumnus who represented ‘everything the school stands for.’ THE BACK-TO-SCHOOL ISSUE FALL 2020 1 You belong at Notre Dame You belong at Notre Dame, a place where students can be faithful, challenged, confident, creative and loved. To schedule a visit or to find out more, go to ndpma.org, or call the admissions office at 248-373-1061. 2 IRISH On the Cover The impressionist portrait of Fr. Jean- portrait was designed to show that he Claude Colin, s.m., was created by was indeed an unconventional man, Sophia Gunterman NDP’17, a graphic a man of many insights, talents and design student at the College for ”colors,” and decidedly, a man of many IRISH Creative Studies (CCS) in Detroit. The dimensions. More on Gunterman is on unconventional approach to Fr. Colin’s page 22. Fall 2020 | Issue 25 IRISH magazine is dedicated to alumni, parents and friends of Notre Dame Preparatory School and Marist 22 Academy and our heritage schools, including Notre Dame A Saintly Educator High School, Pontiac Catholic High School, Oakland Catholic High School, St. Michael High School and Notre Dame’s Fr. Leon discusses the St. Frederick High School. founder of the Society of Mary and his road to sainthood. 4 VICE PRESIDENT FOR What a Year ADVANCEMENT Mark Roberts Head of School Andrew J. -

Received by the Regents February 20, 2020
The University of Michigan Office of Development Unit Report of Gifts Received 4 Year Report as of December 31, 2019 Transactions Dollars Fiscal Year Ended June 30, Fiscal Fiscal Fiscal Year Ended June 30, Fiscal Fiscal YTD YTD YTD YTD Unit 2017 2018 2019 December 31, 2018 December 31, 2019 2017 2018 2019 December 31, 2018 December 31, 2019 A. Alfred Taubman College of Architecture and Urban Planning 890 785 834 469 474 4,761,133 6,664,328 4,858,713 3,905,392 4,367,777 Penny W. Stamps School of Art and Design 565 644 615 366 348 2,052,654 2,133,117 1,795,126 1,236,963 1,466,436 Stephen M. Ross School of Business 8,418 7,897 7,610 4,332 3,801 32,333,848 31,703,408 41,662,544 26,456,778 20,994,441 School of Dentistry 1,822 1,782 1,920 980 896 6,492,684 4,201,101 3,080,617 1,618,436 1,780,580 School of Education 2,412 2,336 2,557 1,375 1,620 5,938,803 8,474,970 11,298,726 4,507,986 3,578,827 College of Engineering 7,961 7,321 7,459 4,051 3,983 29,135,173 33,935,813 31,682,539 17,337,173 12,892,451 School for Environment and Sustainability 929 900 918 501 556 2,691,334 4,469,385 2,220,414 1,433,307 3,301,149 Horace H. Rackham School of Graduate Studies 2,935 2,508 2,617 1,456 1,400 5,531,888 5,010,647 6,083,214 4,218,373 1,010,695 School of Information 1,328 1,446 1,345 757 706 921,261 1,189,629 3,412,544 2,459,072 670,461 School of Kinesiology 975 1,016 988 525 539 1,361,012 1,014,328 1,394,594 460,816 842,877 Law School 5,984 5,800 5,588 2,967 2,972 14,320,487 24,615,924 12,771,100 7,008,304 8,169,805 College of Literature, Science, and the Arts 17,344 17,407 19,014 10,787 10,625 49,141,904 44,983,558 57,532,052 35,592,264 20,851,552 School of Music, Theatre & Dance 4,583 5,221 4,293 2,632 2,440 8,149,703 6,950,036 10,541,563 5,044,782 3,677,925 School of Nursing 1,695 1,693 1,728 972 859 2,891,012 2,638,340 2,999,712 1,589,844 1,764,274 College of Pharmacy 1,097 1,035 984 521 502 3,273,265 3,451,305 1,945,261 1,230,664 1,207,111 School of Public Health 1,735 1,738 1,760 965 980 7,688,432 11,536,036 4,915,632 1,979,543 1,326,949 Gerald R. -

Sheila M. Marcus, M.D. Education and Training Certification and Licensure
Sheila M. Marcus, M.D. Clinical Professor of Psychiatry Section Chief Child and Adolescent Psychiatry Co-Director Infant and Early Childhood Clinic 4250 Plymouth Rd, #2531 Ann Arbor, Michigan 48109-2700 734-764-0245 [email protected] Education and Training University of Michigan B.S. Zoology 9/1975-5/1979 Ann Arbor, Michigan University of Michigan M.D. 9/1979-5/1983 Medical School Ann Arbor, Michigan Internship and Residency, Adult Psychiatry 6/1983-6/1986 University of Michigan Health Systems Ann Arbor, Michigan Fellow in Intensive Outpatient Psychiatry and Substance Abuse 1/1987-3/1987 Mayo Graduate School of Medicine Rochester, Minnesota Adult Psychiatry Residency 6/1987-6/1989 University of Michigan Health Systems Ann Arbor, Michigan Fellow in Child and Adolescent Psychiatry 7/1989-6/1991 University of Michigan Medical School Ann Arbor, Michigan Certification and Licensure Board Certified in Adult Psychiatry 1992 Board Certified in Child and Adolescent Psychiatry 1992 State of Michigan Physician License State of Michigan Controlled Substance License Sheila M. Marcus June 2019 1 Academic, Administrative, and Clinical Appointments Academic Appointments: Lecturer, Department of Psychiatry 9/1991 – 8/1996 University of Michigan Medical School Clinical Assistant Professor 9/1996 – 8/2004 Department of Psychiatry University of Michigan Medical School Clinical Associate Professor 9/2004 – 8/2009 Department of Psychiatry University of Michigan Medical School Clinical Professor 9/2009 – present Department of Psychiatry University of Michigan -

КАРДИОЛОГИЯ С ИЛЛЮСТРАЦИЯМИ НЕТТЕРА NETTER’S CARDIOLOGY 3Rd EDITION
КАРДИОЛОГИЯ С ИЛЛЮСТРАЦИЯМИ НЕТТЕРА NETTER’S CARDIOLOGY 3rd EDITION EDITED BY ILLUSTRATIONS BY George A. Stouffer, MD Frank H. Netter, MD Ernest and Hazel Craige Distinguished Professor of Medicine Chief, Division of Cardiology Physician in Chief, UNC Heart and Vascular Service Line CONTRIBUTING ILLUSTRATORS University of North Carolina at Chapel Hill Chapel Hill, North Carolina Carlos A. G. Machado, MD Marschall S. Runge, MD, PhD John A. Craig, MD Professor of Internal Medicine Dean, University of Michigan Medical School David J. Mascaro, MS Executive Vice President for Medical Affairs Chief Executive Officer, Michigan Medicine Enid Hatton Ann Arbor, Michigan Steven Moon, MA Cam Patterson, MD, MBA Kip Carter, MS, CMI Chancellor University of Arkansas for Medical Sciences Tiffany S. DaVanzo, MA, CMI Little Rock, Arkansas Joseph S. Rossi, MD Associate Professor of Medicine Director, Cardiac Catheterization Laboratory University of North Carolina at Chapel Hill Chapel Hill, North Carolina КАРДИОЛОГИЯ С ИЛЛЮСТРАЦИЯМИ НЕТТЕРА ПЕРЕВОД 3-го ИЗДАНИЯ ДЖОРДЖ А. СТАФФЕР • МАРШАЛЛ С. РУНГЕ КЭМ ПАТТЕРСОН • ДЖОЗЕФ С. РОССИ ИЛЛЮСТРАЦИИ Фрэнка Генри Неттера ПРИ УЧАСТИИ Карлоса А. Мачадо, Джона А. Крэйга, Дэвида Маскаро, Энида Хаттона, Стивена Муна, Кипа Картера, Тиффани С. ДаВанзо ПЕРЕВОД С АНГЛИЙСКОГО П. П. Виноградов Москва, 2021 УДК 616.12 ББК 54.101 К21 Кардиология с иллюстрациями Неттера / Стаффер Джордж К21 А.; Рунге, Маршалл С.; Паттерсон, Кэм; Росси, Джозеф С. и др.; пер с англ. – М.: Издательство Панфилова, 2021. – 644 с.: илл. ISBN 978-5-91839-118-1 Перед Вами «вечная классика» — книга с рисунками легендар- ного Фрэнка Неттера. Авторы дополнили более 500 выдающих- ся иллюстраций современным текстом с ключевой информацией о клинической анатомии и патофизиологии, клинической картине, оптимальной диагностике и тактике ведения пациентов в совре- менной кардиологии. -

Retreat Roster
First Name Last Name Nickname Title Institution / Affiliation Unit/Department Jeffrey Agnoli Jeff Senior T&D Specialist The Ohio State University Research Development Office Grants Administrator/Research Leigh Allen Leigh University of Minnesota Medicinal Chemistry Program Manager Indiana University Purdue University Amjad Alrehaili Amjad Graduate Assistant Office of the Vice President for Research Indianapolis Kate Bak Kate Clinical Research Coordinator University of Minnesota Radiation Oncology Institutional Strategic Initiatives Celine Bauwens Celine University of Toronto Vice-President, Research & Innovation Officer Assistant Director - Grant Kristin Beck Kristin Northern Michigan University Grants & Contracts Development/Training Coordinator Amy Bellas Amy Senior Director University of Michigan Business Engagement Center Maggie Berg Maggie Research Development Manager University of Illinois at Urbana-Champaign Interdisciplinary Health Sciences Institute Karrie Black Karrie Research Administrator University of Michigan Medical School Internal Medicine / Infectious Diseases Kristin Boman Kristin Program Manager University of Minnesota Medicine Crystal Botham Crystal Director Research Development Stanford University Biosciences Programs / Pediatrics Jessica Brassard Jessica Associate Directorate Michigan Technological University Research Development Development Officer - Grant Writer, Mindy Breva Mindy University of Minnesota Foundation University of Minnesota Foundation Foundation Relations Aneesa Buageila Aneesa Research Administrator