CARBAPENEM RESISTANCE from Diagnosis to Outbreak Management TABLE of CONTENTS INTRODUCTION
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Polymyxin B in Combination with Doripenem Against Heteroresistant Acinetobacter Baumannii: Pharmacodynamics of New Dosing Strategies
J Antimicrob Chemother 2016; 71: 3148–3156 doi:10.1093/jac/dkw293 Advance Access publication 3 August 2016 Polymyxin B in combination with doripenem against heteroresistant Acinetobacter baumannii: pharmacodynamics of new dosing strategies Gauri G. Rao1,2, Neang S. Ly1,2,Ju¨rgen B. Bulitta1,3, Rachel L. Soon1,2, Marie D. San Roman1,2, Patricia N. Holden1,2, Cornelia B. Landersdorfer4, Roger L. Nation4, Jian Li4, Alan Forrest1,5 and Brian T. Tsuji1,2* 1Laboratory for Antimicrobial Pharmacodynamics, School of Pharmacy and Pharmaceutical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, USA; 2The New York State Center of Excellence in Bioinformatics & Life Sciences, University at Buffalo, The State University of New York, Buffalo, NY, USA; 3Center for Pharmacometrics and Systems Pharmacology, College of Pharmacy, University of Florida, Orlando, FL, USA; 4Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, Australia; 5Eshelman School of Pharmacy, The University of North Carolina at Chapel Hill, NC, USA *Corresponding author. Tel: +1-716-881-7543; Fax: +1-716-849-6890; E-mail: [email protected] Received 13 October 2015; returned 3 January 2016; revised 14 June 2016; accepted 19 June 2016 Objectives: Polymyxin B is being increasingly utilized as a last resort against resistant Gram-negative bacteria. We examined the pharmacodynamics of novel dosing strategies for polymyxin B combinations to maximize efficacy and minimize the emergence of resistance and drug exposure against Acinetobacter baumannii. Methods: The pharmacodynamics of polymyxin B together with doripenem were evaluated in time–kill experi- ments over 48 h against 108 cfu/mL of two polymyxin-heteroresistant A. -

Cusumano-Et-Al-2017.Pdf
International Journal of Infectious Diseases 63 (2017) 1–6 Contents lists available at ScienceDirect International Journal of Infectious Diseases journal homepage: www.elsevier.com/locate/ijid Rapidly growing Mycobacterium infections after cosmetic surgery in medical tourists: the Bronx experience and a review of the literature a a b c b Lucas R. Cusumano , Vivy Tran , Aileen Tlamsa , Philip Chung , Robert Grossberg , b b, Gregory Weston , Uzma N. Sarwar * a Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York, USA b Division of Infectious Diseases, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York, USA c Department of Pharmacy, Nebraska Medicine, Omaha, Nebraska, USA A R T I C L E I N F O A B S T R A C T Article history: Background: Medical tourism is increasingly popular for elective cosmetic surgical procedures. However, Received 10 May 2017 medical tourism has been accompanied by reports of post-surgical infections due to rapidly growing Received in revised form 22 July 2017 mycobacteria (RGM). The authors’ experience working with patients with RGM infections who have Accepted 26 July 2017 returned to the USA after traveling abroad for cosmetic surgical procedures is described here. Corresponding Editor: Eskild Petersen, Methods: Patients who developed RGM infections after undergoing cosmetic surgeries abroad and who ?Aarhus, Denmark presented at the Montefiore Medical Center (Bronx, New York, USA) between August 2015 and June 2016 were identified. A review of patient medical records was performed. Keywords: Results: Four patients who presented with culture-proven RGM infections at the sites of recent cosmetic Mycobacterium abscessus complex procedures were identified. -

General Items
Essential Medicines List (EML) 2019 Application for the inclusion of imipenem/cilastatin, meropenem and amoxicillin/clavulanic acid in the WHO Model List of Essential Medicines, as reserve second-line drugs for the treatment of multidrug-resistant tuberculosis (complementary lists of anti-tuberculosis drugs for use in adults and children) General items 1. Summary statement of the proposal for inclusion, change or deletion This application concerns the updating of the forthcoming WHO Model List of Essential Medicines (EML) and WHO Model List of Essential Medicines for Children (EMLc) to include the following medicines: 1) Imipenem/cilastatin (Imp-Cln) to the main list but NOT the children’s list (it is already mentioned on both lists as an option in section 6.2.1 Beta Lactam medicines) 2) Meropenem (Mpm) to both the main and the children’s lists (it is already on the list as treatment for meningitis in section 6.2.1 Beta Lactam medicines) 3) Clavulanic acid to both the main and the children’s lists (it is already listed as amoxicillin/clavulanic acid (Amx-Clv), the only commercially available preparation of clavulanic acid, in section 6.2.1 Beta Lactam medicines) This application makes reference to amendments recommended in particular to section 6.2.4 Antituberculosis medicines in the latest editions of both the main EML (20th list) and the EMLc (6th list) released in 2017 (1),(2). On the basis of the most recent Guideline Development Group advising WHO on the revision of its guidelines for the treatment of multidrug- or rifampicin-resistant (MDR/RR-TB)(3), the applicant considers that the three agents concerned be viewed as essential medicines for these forms of TB in countries. -

Comparative Effects of Cefpirome (Hr 810) and Other Cephalosporins on Experimentally Induced Pneumonia in Mice
VOL. XXXIX NO. 7 THE JOURNAL OF ANTIBIOTICS 971 COMPARATIVE EFFECTS OF CEFPIROME (HR 810) AND OTHER CEPHALOSPORINS ON EXPERIMENTALLY INDUCED PNEUMONIA IN MICE N. KLESEL, D. ISERT, M. LIMBERT, G. SEIBERT, I. WINKLER and E. SCHRINNER Hoechst Aktiengesellschaft, Dept. of Chemotherapy, 6230 Frankfurt a. M. 80, FRG (Received for publication March 24, 1986) The chemotherapeutic efficacy of cefpirome (HR 810), a new polar aminothiazolyl- cephalosporin and that of ceftazidime, cefotaxime, cefoperazone, latamoxef and cefodizime were examined against experimental pneumonia caused by Klebsiella pneammlliae DT-S in mice. When compared in terms of MIC values against the infecting organism and the phar- macokinetic pattern, cefpirome showed equal activity and a similar pharmacokinetic behavior to ceftazidime and cefotaxime in mice. Trials to assess the bactericidial activity in vhro, however, showed that cefpirome displayed a more marked bactericidal effect in pneumonic mice than the other cephalosporins tested. Only cefodizime, a cephalosporin with extremely high and prolonged blood and tissue levels in experimental animals exerted chemotherapeutic effects similar to cefpirome. After cefpirome or cefodizime medication (50 mg/kg), the viable counts in the lungs of experimental animals fell steadily to 1/10,000 of the pretreatment level and, in contrast to the reference compounds, no regrowth of the challenge organisms could be observed with both drugs. Moreover, with ED.-,,,,sranging from 1.1 to 59.1 mg/kg in treatment studies, cefpirome as well as cefodizime were two to ten times more effective than ceftazidime and cefotaxime, whereas cefoperazone and latamoxef were considerably less effective. Cefpirome (3-((2,3-cyclopenteno-I-pyridium)methyl)-7-(2-syn-methoximino-2-(2-aminothiazol-4- yl)acetamido)ceph-3-em-4-carboxylate, HR 810) is a new semi-synthetic parenteral cephalosporin antibiotic with a broad spectrum of antibacterial activity in vitro and in rivo. -
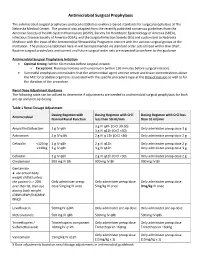
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Piperacillin-Tazobactam Allergies: an Exception to Usual Penicillin Allergy
Allergy Asthma Immunol Res. 2021 Mar;13(2):284-294 https://doi.org/10.4168/aair.2021.13.2.284 pISSN 2092-7355·eISSN 2092-7363 Original Article Piperacillin-Tazobactam Allergies: An Exception to Usual Penicillin Allergy Jane CY Wong ,1 Elaine YL Au ,2 Heather HF Yeung ,2 Chak-Sing Lau ,1 Philip Hei Li 1* 1Division of Rheumatology and Clinical Immunology, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong 2Division of Clinical Immunology, Department of Pathology, The University of Hong Kong, Queen Mary Hospital, Hong Kong Received: Apr 21, 2020 Revised: Jul 20, 2020 ABSTRACT Accepted: Jul 26, 2020 Purpose: The majority of penicillin allergy labels are false, and skin tests (ST) have high Correspondence to negative predictive value (NPV) of up to 90%. Piperacillin-tazobactam (PT) allergy has been Philip Hei Li, MBBS, FHKCP suspected to be an exception to this, but existing literature is scarce. We investigate the Division of Rheumatology & Clinical Immunology, Department of Medicine, The epidemiology, clinical characteristics, testing outcomes and predictive value of ST in patients University of Hong Kong, Queen Mary Hospital, referred for suspected PT allergies. 102 Pokfulam Road, Hong Kong. Methods: The records of all patients referred for suspected PT allergy testing and Tel: +852-2255-3348 prescription rates of PT in all Hong Kong public hospitals (2015–2019) were analyzed. Fax: +852-2816-2863 Results: There was an increase in both PT prescriptions and number of newly reported E-mail: [email protected] PT allergies between 2015 and 2019. The majority (91.1%) of patients with suspected PT Copyright © 2021 The Korean Academy of allergy had at least 1 underlying medical co-morbidity or immunosuppressant use leading to Asthma, Allergy and Clinical Immunology • increased risk of infections. -

Antimicrobial Stewardship Guidance
Antimicrobial Stewardship Guidance Federal Bureau of Prisons Clinical Practice Guidelines March 2013 Clinical guidelines are made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant these guidelines for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Consult the BOP Clinical Practice Guidelines Web page to determine the date of the most recent update to this document: http://www.bop.gov/news/medresources.jsp Federal Bureau of Prisons Antimicrobial Stewardship Guidance Clinical Practice Guidelines March 2013 Table of Contents 1. Purpose ............................................................................................................................................. 3 2. Introduction ...................................................................................................................................... 3 3. Antimicrobial Stewardship in the BOP............................................................................................ 4 4. General Guidance for Diagnosis and Identifying Infection ............................................................. 5 Diagnosis of Specific Infections ........................................................................................................ 6 Upper Respiratory Infections (not otherwise specified) .............................................................................. -

Susceptibility of Pseudomonas Cepacia Isolated from Children with Cystic Fibrosis1
003 1-3998/86/2011-1174$02.00/0 PEDIATRIC RESEARCH Vol. 20, No. 1 1, 1986 Copyright O 1986 International Pediatric Research Foundation, Inc. Printed in (I.S.A. Decreased Baseline P-Lactamase Production and Inducibility associated with Increased Piperacillin Susceptibility of Pseudomonas cepacia Isolated from Children with Cystic Fibrosis1 CLAUDIO CHIESA,~PAULINE H. LABROZZI, AND STEPHEN C. ARONOFF Department of Pediatrics [C.C., P.H.L., S.C.A.], Case- Western Reserve University School ofMedicine and the Division of Pediatric Infectious Diseases [S.C.A.], Rainbow Babies and Children's Hospital, Cleveland, Ohio ABSTRACT. The incidence of pulmonary infections in creased since 1978 and is now recovered from 20% of patients children with cystic fibrosis caused by Pseudomonas ce- in some centers (3, 4). pacia, an organism which may possess an inducible 8- Sputum isolates of P. cepacia from children with cystic fibrosis lactamase, has increased since 1978. Seven of 13 sputum are resistant to most P-lactam agents in vitro. In an in vitro study isolates of P. cepacia from children with cystic fibrosis comparing the susceptibilities of 62 consecutive sputum isolates, were classified as inducible by quantitative enzyme produc- concentrations of 64 pg/ml of aztreonam and piperacillin were tion following preincubation with 100, 200, or 400 pg/ml required to inhibit 79 and 87.1 % of the bacterial population, of cefoxitin. The recovery of inducible strains tended to be respectively; 90% of the isolates were inhibited by 8 pg/ml of associated with recent ceftazidime therapy. Susceptibility ceftazidime (5). In vitro, ceftazidime has proven more active to aztreonam, ceftazidime, and piperacillin alone or com- against P. -

New Β-Lactamase Inhibitor Combinations: Options for Treatment; Challenges for Testing
MEDICAL/SCIENTIFIC AffAIRS BULLETIN New β-lactamase Inhibitor Combinations: Options for Treatment; Challenges for Testing Background The β-lactam class of antimicrobial agents has played a crucial role in the treatment of infectious diseases since the discovery of penicillin, but β–lactamases (enzymes produced by the bacteria that can hydrolyze the β-lactam core of the antibiotic) have provided an ever expanding threat to their successful use. Over a thousand β-lactamases have been described. They can be divided into classes based on their molecular structure (Classes A, B, C and D) or their function (e.g., penicillinase, oxacillinase, extended-spectrum activity, or carbapenemase activity).1 While the first approach to addressing the problem ofβ -lactamases was to develop β-lactamase stable β-lactam antibiotics, such as extended-spectrum cephalosporins, another strategy that has emerged is to combine existing β-lactam antibiotics with β-lactamase inhibitors. Key β-lactam/β-lactamase inhibitor combinations that have been used widely for over a decade include amoxicillin/clavulanic acid, ampicillin/sulbactam, and pipercillin/tazobactam. The continued use of β-lactams has been threatened by the emergence and spread of extended-spectrum β-lactamases (ESBLs) and more recently by carbapenemases. The global spread of carbapenemase-producing organisms (CPOs) including Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii, limits the use of all β-lactam agents, including extended-spectrum cephalosporins (e.g., cefotaxime, ceftriaxone, and ceftazidime) and the carbapenems (doripenem, ertapenem, imipenem, and meropenem). This has led to international concern and calls to action, including encouraging the development of new antimicrobial agents, enhancing infection prevention, and strengthening surveillance systems. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -

Carbapenem-Resistant Acinetobacter Threat Level Urgent
CARBAPENEM-RESISTANT ACINETOBACTER THREAT LEVEL URGENT 8,500 700 $281M Estimated cases Estimated Estimated attributable in hospitalized deaths in 2017 healthcare costs in 2017 patients in 2017 Acinetobacter bacteria can survive a long time on surfaces. Nearly all carbapenem-resistant Acinetobacter infections happen in patients who recently received care in a healthcare facility. WHAT YOU NEED TO KNOW CASES OVER TIME ■ Carbapenem-resistant Acinetobacter cause pneumonia Continued infection control and appropriate antibiotic use and wound, bloodstream, and urinary tract infections. are important to maintain decreases in carbapenem-resistant These infections tend to occur in patients in intensive Acinetobacter infections. care units. ■ Carbapenem-resistant Acinetobacter can carry mobile genetic elements that are easily shared between bacteria. Some can make a carbapenemase enzyme, which makes carbapenem antibiotics ineffective and rapidly spreads resistance that destroys these important drugs. ■ Some Acinetobacter are resistant to nearly all antibiotics and few new drugs are in development. CARBAPENEM-RESISTANT ACINETOBACTER A THREAT IN HEALTHCARE TREATMENT OVER TIME Acinetobacter is a challenging threat to hospitalized Treatment options for infections caused by carbapenem- patients because it frequently contaminates healthcare resistant Acinetobacter baumannii are extremely limited. facility surfaces and shared medical equipment. If not There are few new drugs in development. addressed through infection control measures, including rigorous -

Time-Series Analysis of the Relationship of Antimicrobial Use and Hand Hygiene Promotion with the Incidence of Healthcare-Associated Infections
The Journal of Antibiotics (2012) 65, 311–316 & 2012 Japan Antibiotics Research Association All rights reserved 0021-8820/12 www.nature.com/ja ORIGINAL ARTICLE Time-series analysis of the relationship of antimicrobial use and hand hygiene promotion with the incidence of healthcare-associated infections Yuan-Ti Lee1,2,3, Shiuan-Chih Chen1,2,4, Meng-Chih Lee1,2,4, Hung-Chang Hung1,2,5, Huey-Jen Huang3,6, Hui-Chih Lin1,7, Der-Jinn Wu1,2,3 and Shih-Ming Tsao1,2,3 We analyzed the effect of antimicrobial use and implementation of a hand hygiene program on the incidence of healthcare- associated infections (HAIs) and healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) infections at the Chung Shan Medical University Hospital (Taichung, Taiwan). Monthly data were retrospectively reviewed from January 2004 to December 2010. Use of antimicrobials and alcohol-based hand cleaner were separately regressed against the incidences of HAIs and HA-MRSA infections. Infection incidence was expressed as persons per 1000 patient days (PDs), monthly use of i.v. antibiotics was expressed as defined daily doses per 1000 PDs and monthly alcohol-based hand cleaner use was expressed as bottle per 1000 PDs. Multivariate analysis indicated that use of hand cleaner was associated with reduced incidence of HAIs (P ¼ 0.0001) and HA-MRSA infections (Po0.0001). Time-series analysis indicated that increased use of hand cleaner was significantly associated with significant decreases in the incidences of HAIs and HA-MRSA infections. Total antibiotic use had no significant effect on HAIs, but was associated with more HA-MRSA infections.