Femtoliter Volumetric Pipette and Flask Utilizing Nanofluidics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Glassware N Edition No
Laboratory Glassware n Edition No. 2 n Index Introduction 3 Ground joint glassware 13 Volumetric glassware 53 General laboratory glassware 65 Alphabetical index 76 Índice alfabético 77 Index Reference index 78 [email protected] Scharlau has been in the scientific glassware business for over 15 years Until now Scharlab S.L. had limited its sales to the Spanish market. However, now, coinciding with the inauguration of the new workshop next to our warehouse in Sentmenat, we are ready to export our scientific glassware to other countries. Standard and made to order Products for which there is regular demand are produced in larger Scharlau glassware quantities and then stocked for almost immediate supply. Other products are either manufactured directly from glass tubing or are constructed from a number of semi-finished products. Quality Even today, scientific glassblowing remains a highly skilled hand craft and the quality of glassware depends on the skill of each blower. Careful selection of the raw glass ensures that our final products are free from imperfections such as air lines, scratches and stones. You will be able to judge for yourself the workmanship of our glassware products. Safety All our glassware is annealed and made stress free to avoid breakage. Fax: +34 93 715 67 25 Scharlab The Lab Sourcing Group 3 www.scharlab.com Glassware Scharlau glassware is made from borosilicate glass that meets the specifications of the following standards: BS ISO 3585, DIN 12217 Type 3.3 Borosilicate glass ASTM E-438 Type 1 Class A Borosilicate glass US Pharmacopoeia Type 1 Borosilicate glass European Pharmacopoeia Type 1 Glass The typical chemical composition of our borosilicate glass is as follows: O Si 2 81% B2O3 13% Na2O 4% Al2O3 2% Glass is an inorganic substance that on cooling becomes rigid without crystallising and therefore it has no melting point as such. -
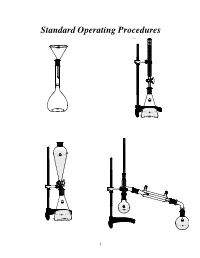
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

Environmental Protection Agency Pt. 63, App. A
Environmental Protection Agency Pt. 63, App. A APPENDIX A TO PART 63—TEST METHODS posed as an alternative test method to meet an applicable requirement or in the absence METHOD 301—FIELD VALIDATION OF POLLUT- of a validated method. Additionally, the val- ANT MEASUREMENT METHODS FROM VARIOUS idation procedures of Method 301 are appro- WASTE MEDIA priate for demonstration of the suitability of alternative test methods under 40 CFR parts USING METHOD 301 59, 60, and 61. If, under 40 CFR part 63 or 60, 1.0 What is the purpose of Method 301? you choose to propose a validation method other than Method 301, you must submit and 2.0 What approval must I have to use Method obtain the Administrator’s approval for the 301? candidate validation method. 3.0 What does Method 301 include? 2.0 What approval must I have to use Method 301? 4.0 How do I perform Method 301? If you want to use a candidate test method REFERENCE MATERIALS to meet requirements in a subpart of 40 CFR part 59, 60, 61, 63, or 65, you must also request 5.0 What reference materials must I use? approval to use the candidate test method according to the procedures in Section 16 of SAMPLING PROCEDURES this method and the appropriate section of 6.0 What sampling procedures must I use? the part (§ 59.104, § 59.406, § 60.8(b), § 61.13(h)(1)(ii), § 63.7(f), or § 65.158(a)(2)(iii)). 7.0 How do I ensure sample stability? You must receive the Administrator’s writ- ten approval to use the candidate test meth- DETERMINATION OF BIAS AND PRECISION od before you use the candidate test method to meet the applicable federal requirements. -

Environmental Chemistry Method Methoxyfenozide & Degradates In
Dow AgroSciences LLC Study ID: 110356 Page 12 Method Validation Study for the Detennination of Residues of Methoxyfenozide and its A-ring • Phenol Metabolite and B-ring Mono Acid Metabolite in Surface Water, Ground Water and Drinking Water by Liquid Chromatography with Tandem Mass Spectrometry INTRODUCTION J This method is applicable for the quantitative detennination of residues of methoxyfenozide and its A-ring phenol metabolite and B-ring mono acid metabolite in surface, ground and drinking water. The method was validated over the concentration range of 0.05-1.0 µg/L with a validated limit of quantitation of 0.05 µg/L. Common names, chemical names, and molecular formulas for the analytes are given in Table I. This study was conducted to fulfill data requirements outlined in the EPA Residue Chemistry Test Guidelines, OPPTS 850. 7100 (/). The validation will also comply with the requirements of EU Council Regulation (EC) No. 1107/2009 with particular regard to Section 3 of SANCO/3029/99 rev.4 and Section 3 of SANCO/825/00 rev.8.1 as well as PMRA Regulatory Directive Dir98-02 (2-4). The validation was conducted following Dow AgroSciences SOP ECL-24 with exceptions noted in the protocol or by protocol amendment. Method Principle Residues of methoxyfenozide, its A-ring phenol metabolite and its B-ring mono acid metabolite • are extracted from water samples by taking a l 0.0-mL aliquot and placing in an I I -dram (45-mL) glass vial equipped with a PTFE-lined cap or a 50-mL polypropylene centrifuge tube equipped with a cap. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

Q & a – Accuracy and Precision
Food Analysis – FScN 4312W Laboratory: Assessment of Accuracy and Precision Key to Questions 1. Theoretically, how are standard deviation, coefficient of variation, mean, percent relative error, and 95% confidence interval affected by: (1) more replicates, and (2) a larger size of measurement? Was this evident in looking at the actual results obtained using the volumetric pipettes and the buret, with n = 3 vs n = 6, and with 1mL vs 10mL? Ans: (1) As the sample size increases (more replicates) - Calculated mean approaches the true mean - Standard deviation is inversely proportional to the square root of sample size, hence it decreases - CV decreases, as standard deviation approaches 0 for larger sample size - % Relative error decreases as calculated mean approaches true mean - 95% confidence interval narrows down (2) Larger size of measurement - Mean is close to true mean - Standard deviation might be larger due to larger size - CV decreases - % Relative error decreases, as calculated mean is close to true mean - 95% confidence interval narrows down Not all of the above assumptions were confirmed in this experiment according to the data observed. One reason could be that the sample size was very small (n = 3 and n = 6), to notice significant differences, another reason could be human error and variation from one person’s measuring technique to another. 2. Why are percent relative error and coefficient of variation used to compare the accuracy and precision, respectively, of the volumes from pipetting/dispensing 1 and 10mL with the volumetric pipettes and buret in parts 2 and 3, rather than simply the mean and standard deviation, respectively? Ans: Mean calculated from the readings gives the calculated mean, which may differ from the true mean. -
![32-9-2.5.2 Stop TB Global Drug Facility Diagnostics Catalog [.Pdf]](https://docslib.b-cdn.net/cover/2786/32-9-2-5-2-stop-tb-global-drug-facility-diagnostics-catalog-pdf-1302786.webp)
32-9-2.5.2 Stop TB Global Drug Facility Diagnostics Catalog [.Pdf]
OCTOBER 2019 DIAGNOSTICS CATALOG GLOBAL DRUG FACILITY (GDF) PHOTO: MAKA AKHALAIA PHOTO: Ensuring an uninterrupted supply of quality-assured, affordable tuberculosis (TB) medicines and diagnostics to the world. stoptb.org/gdf Stop TB Partnership | Global Drug Facility Global Health Campus – Chemin du Pommier 40 1218 Le Grand-Saconnex | Geneva, Switzerland Email: [email protected] Last verion's date: 08 October 2019. Stop TB Partnership/Global Drug Facility licensed this product under an Attribution-NonCommercial-NoDerivatives 4.0 International License. (CC BY-NC-ND 4.0) https://creativecommons.org/licenses/by-nc-nd/4.0/legalcode GLOBAL DRUG FACILITY DIAGNOSTICS CATALOG OCTOBER 2019 GDF is the largest global provider of quality-assured tuberculosis (TB) medicines, diagnostics, and laboratory supplies to the public sector. Since 2001, GDF has facilitated access to high-quality TB care in over 130 countries, providing treatments to over 30 million people with TB and procuring and delivering more than $200 million worth of diagnostic equipment. As a unit of the Stop TB Partnership, GDF provides a full range of quality-assured products to meet the needs of any TB laboratory globally. GDF provides more than 500 diagnostics products, including the latest WHO-approved TB diagnostic devices and reagents, together with the consumables and ancillary devices required to ensure a safe working environment. These products cater to all levels of laboratories, ranging from peripheral health centers to centralized reference laboratories, and provide countries with the latest WHO-recommended technologies for detecting TB and drug resistance. To place an order for any diagnostics product, please follow the step-by-step guide available on the GDF website. -

Laboratory Equipment Used in Filtration
KNOW YOUR LAB EQUIPMENTS Test tube A test tube, also known as a sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom. Beakers Beakers are used as containers. They are available in a variety of sizes. Although they often possess volume markings, these are only rough estimates of the liquid volume. The markings are not necessarily accurate. Erlenmeyer flask Erlenmeyer flasks are often used as reaction vessels, particularly in titrations. As with beakers, the volume markings should not be considered accurate. Volumetric flask Volumetric flasks are used to measure and store solutions with a high degree of accuracy. These flasks generally possess a marking near the top that indicates the level at which the volume of the liquid is equal to the volume written on the outside of the flask. These devices are often used when solutions containing dissolved solids of known concentration are needed. Graduated cylinder Graduated cylinders are used to transfer liquids with a moderate degree of accuracy. Pipette Pipettes are used for transferring liquids with a fixed volume and quantity of liquid must be known to a high degree of accuracy. Graduated pipette These Pipettes are calibrated in the factory to release the desired quantity of liquid. Disposable pipette Disposable transfer. These Pipettes are made of plastic and are useful for transferring liquids dropwise. Burette Burettes are devices used typically in analytical, quantitative chemistry applications for measuring liquid solution. Differing from a pipette since the sample quantity delivered is changeable, graduated Burettes are used heavily in titration experiments. -

TOP-014, Technical Operating Procedure for Spectrophotometric
CENTER FOR NUCLEAR WASTE Proc. T0P-014 REGULATORY ANALYSES Revision ° TECHNICAL OPERATING PROCEDURE Page I of 11 Title TECHNICAL OPERATING PROCEDURE FOR SPECTROPHOTOMETRIC DETERMINATION OF SILICA EFFECTIVITY AND APPROVAL Revision n of this procedure became effective on 03/04/91 This procedure consists of the pages and changes listed below. Page No. Change Date Effective ALL 03/04/91 Superseaes Procedure No. None Approvals Written By Technical Revie / CENTER FOR NUCLEAR WASTE Proc.TOP-014 REGULATORY ANALYSES Revision _ TECHNICAL OPERATING PROCEDURE Page 2 of 1il TOP-014 TECHNICAL OPERATING PROCEDURE FOR SPECTROPHOTOMETRIC DETERMINATION OF SILICA 1. PURPOSE The purpose of this procedure is to describe a general method for the spectrophotometric determination of silica (Si02) in aqueous solutions. This procedure will be used for aqueous solutions generated by geochemical experiments involving silicate minerals (e.g., zeolites and feldspars) but may also be used for analyses of water samples collected from laboratory experiments or in the field. This procedure implements the requirements of CQAM Section 3. 2. APPLICABLE DOCUMENT The following document forms a part of this procedure, as applicable: Operator's Manual for Milton Roy Spectronic 1201 Spectrophotometer (1988) 3. RESPONSIBILITY (1) The Geosciences Element Manager shall be responsible for the development and maintainance of this procedure. (2) The cognizant Principal Investigator shall be responsible for the implementation of this procedure. (3) Personnel performing tasks described in this procedure are responsible for complying with its requirements. 4. EQUIPMENT (1) Milton Roy Spectronic 1201 Spectrophotometer with microprocessor-controlled functions and features including a linear curve fit test mode or equivalent. -

Chemistry 50 and 51 Laboratory Manual General Information
Chemistry 50 and 51 Laboratory Manual General Information Mt. San Antonio College Chemistry Department 2019 - 2020 TABLE OF CONTENTS PREFACE………………………………………………………………………..………..… 1 GENERAL INFORMATION Safety………………..………………………..…………………………………INFORMATION….……… 3 Equipment……………………………………..……………………………….………….. 9 Techniques………………………………………………………………………………… 13 Heating……………………………………...……………………………….…..….… 13 Cleaning and Labeling Glassware……….……………………….........……........…... 14 Reading Analog Scales…………………………………………………..……….…... 14 Volumetric Flasks………………………………………………………..……….…... 15 Graduated Cylinders.………………………………………………………………..… 15 Volumetric Pipets……………………………………..…………………………..…... 16 Graduated Pipets……………….……………………..………………………….…… 16 Burets………………………….………………..………………..………..….………. 18 Analytical Balances…………………………………………………….……….…….. 19 Solution Preparation…………………………………………..……………….….…... 20 Percent Concentration....……………………………………………….……….…….. 20 Molarity……………………………………………………………………………….. 21 Dilution……………………………………………………………...………………… 22 Titration ………………………………………………………………....…………..... 23 Vacuum Filtration……………………….…………………………………..…….…… 24 Spectrophotometry and Beer’s Law…………………………………..…………..…... 25 Measurement of pH………………………………………………….….….……..…... 27 Pasco Spectrometer……………………………………..…………………...….…… 28 Vernier Go Direct Sensors ………………………..…………………...….………….. 31 Notebook…………………………………………………………….….………..……...... 35 Precision and Accuracy……………………………………………………….………....... 37 Spreadsheet and Graphing with Excel…..…………………..…………..……………....... 46 EXPERIMENTS PREFACE The laboratory -

List of Equipment
Mfg & Exporters: All types of Turnkey Projects, , Beverages, Mineral Water plant & OEM Spares List Of Equipment SR.NO Equipment Qty 1. Autoclave 1 Vertical Complete Heavy gauze with pressure gauze, Safety Valve, Stream Release Valve Size 30 X 30 cm 2. Digital Hot Air Ageing oven 1 Size 12” x 12” x 12” made of MS powder coated. Make- HB 3. Digital pH System : 1 Make- HB 4. Digital Incubator Bacteriological 2 12” X 12” X 12” made of MS powder coated. Make- HB 5. Water bath double walled 1 inner chamber of SS outer M.S powder coated with 6 hole with thermostatic control Make- HB 6. INNOCULATION CHAMBER, M.S POWDER COATED WITH 1 UV & 1 FLOROCENT LIGHT INBUILT 1 Make- HB 7. Automatic Hot Plate with 8 inch dia with Thermostat 1 Make-HB 8. Digital Spectrophotometer 1 Make- HB 9. Digital Turbidity meter 1 Make- HB 10. Digital Colony Counter 1 Make- HB 11. S.S. Filter holder 47 mm 1 (complete S.S) 12. Microscope student 1 Make-Labron 13. Magnetic Stirrer with Hot Plate with 1 lit capacity Top made of SS with Teflon Bed 1 Make- HB 14. Centrifuge ( 4 holders) 1 Make- HB 15. Single pan Balance ( pocket type) max: 0-500 gm, L.C: 0.1 gm 1 16. High pressure vacuum Pump 1 17. BOD Incubators inner complete S.S outer MS powder coated.( heavy) 2 Make – HB 18. Heating Mental 1 lit capacity 1 Make- HB 19. S.S distillation Unit 1 Address: 140, Tribhuvan Estate opp Road No 11 Kathwada GIDC Contact: +91 9726943972 Email: [email protected] Mfg & Exporters: All types of Turnkey Projects, , Beverages, Mineral Water plant & OEM Spares List Of Chemicals Sr. -

J-Sil Price List
An ISO 9001 : 2008 Certified Company TM J-SIL LABORATORY GLASSWARE Made from Borosilicate 3.3 Glass PRICE LIST 1st April, 2015 Manufactured by : J-SIL SCIENTIFIC INDUSTRIES MFRS. OF : SCIENTIFIC & LABORATORY GLASS APPARATUS 48-B, INDUSTR IAL ESTATE, NUNHAI, AGRA-282006 (INDIA) Phone : 2281967, 2281887 l Fax : 0562-2280186 E-mail : jsil @sancharnet.in, [email protected] Web : www.j-sil.com LABORATORY GLASS APPARATUS J-SIL 51. Chromatography Graduated, Screw Vials 9 mm wide Opening Type Cap Pkt Price/ Pkt A Clear Glass 2ml 100 PC 650.00 B Amber Glass 2ml 100 PC 700.00 56. Screw Cap with Septa for 9 mm Screw Vials (Closure) Cap Septa Pkt Price/ Pkt A Blue Non Slit 100 PC 750.00 B Blue Pre-Slit 100 PC 775.00 61. Clear Glass Screw Vials 9mm with Screw Blue Cap & PTFE-Silicon Septa (Combo) Cap Septa Pkt Price/ Pkt A 2 ml Non Slit 100 PC 1350.00 B 2 ml Pre-Slit 100 PC 1400.00 66. Amber Clear Glass Screw Vials 9mm with Screw Blue Cap & PTFE-Silicon Septa (Combo) Cap Septa Pkt Price/ Pkt A 2 ml Non Slit 100 PC 1400.00 B 2 ml Pre-Slit 100 PC 1450.00 LABORATORY GLASS APPARATUS J-SIL 71. Nylon Syringe Filter (Non Sterile) Packed in Transparent Plastic Jar DIA MICRON Pkt Price/ Pkt A 13 mm 0.22µm 100 1800.00 B 13 mm 0.45µm 100 1800.00 C 25 mm 0.22µm 100 1850.00 D 25 mm 0.45µm 100 1850.00 76. PVDF Syringe Filter (Non Sterile) Packed in Transparent Plastic Jar DIA MICRON Pkt Price/ Pkt A 13 mm 0.22µm 100 2800.00 B 13 mm 0.45µm 100 2800.00 C 25 mm 0.22µm 100 2850.00 D 25 mm 0.45µm 100 2850.00 81.