Cryptic Diversity in Common Mustached Bats Pteronotus Cf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of Mammals 61
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORCHAPTER SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Classification © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 4 NOT FORof SALE MammalsOR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION. 2ND PAGES 9781284032093_CH04_0060.indd 60 8/28/13 12:08 PM CHAPTER 4: Classification of Mammals 61 © Jones Despite& Bartlett their Learning,remarkable success, LLC mammals are much less© Jones stress & onBartlett the taxonomic Learning, aspect LLCof mammalogy, but rather as diverse than are most invertebrate groups. This is probably an attempt to provide students with sufficient information NOT FOR SALE OR DISTRIBUTION NOT FORattributable SALE OR to theirDISTRIBUTION far greater individual size, to the high on the various kinds of mammals to make the subsequent energy requirements of endothermy, and thus to the inabil- discussions of mammalian biology meaningful. -

Isolation and Characterization of Microsatellite Marker Loci in The
Journal of Genetics, Vol. 97, No. 5, December 2018, pp. 1179–1183 © Indian Academy of Sciences https://doi.org/10.1007/s12041-018-1012-y RESEARCH ARTICLE Isolation and characterization of microsatellite marker loci in the Wagner’s mustached bat Pteronotus psilotis (Chiroptera: Mormoopidae) and cross- amplification in other related species A. MÉNDEZ-RODRÍGUEZ1, R. LÓPEZ-WILCHIS1∗, A. SERRATO DÍAZ2, J. JUSTE3,4, M. A. DEL RÍO-PORTILLA5 and L. M. GUEVARA-CHUMACERO1∗ 1Departamento de Biología, and 2Departamento de Hidrobiología, Universidad Autónoma Metropolitana Iztapalapa, Avenida San Rafael Atlixco No. 186, Col. Vicentina. Del. Iztapalapa, C.P. 09340 Ciudad de México, Mexico 3Estación Biológica de Doñana, C.S.I.C., Avda. Américo Vespucio 26, 41092 Sevilla, Spain 4CIBER de Epidemiología y Salud Pública, CIBERESP, Madrid, Spain 5Departamento de Acuicultura, Centro de Investigación Científica y de Educación Superior de Ensenada, B.C., Carretera Ensenada-Tijuana #3918, Zona Playitas, Ensenada, B. C., C.P. 22860, Mexico ∗ For correspondence. E-mail: R. López-Wilchis, [email protected]; L. M. Guevara-Chumacero, [email protected]. Received 20 November 2017; revised 20 April 2018; accepted 25 April 2018; published online 29 October 2018 Abstract. Pteronotus psilotis, a mormoopid bat, is an insectivorous, gregarious and strict cave-dwelling species that is found areas between the sea level and an elevation of about 1000 masl. This species is present in diverse habitats ranging from rain forest to dry deciduous forest. Nine microsatellite loci were developed for Wagner’s mustached bat, Pteronotus psilotis using the next-generation sequencing approach, and their utility for population genetics studies was assessed. -

Diet and the Evolution of Digestion and Renal Function in Phyllostomid Bats
Zoology 104 (2001): 59–73 © by Urban & Fischer Verlag http://www.urbanfischer.de/journals/zoology Diet and the evolution of digestion and renal function in phyllostomid bats Jorge E. Schondube1,*, L. Gerardo Herrera-M.2 and Carlos Martínez del Rio1 1Department of Ecology and Evolutionary Biology, University of Arizona, Tucson 2Instituto de Biología, Departamento de Zoología, Universidad Nacional Autónoma de México, México Received: April 2, 2001 · Accepted: April 12, 2001 Abstract Bat species in the monophyletic family Phyllostomidae feed on blood, insects, small vertebrates, nectar, fruit and complex omnivorous mixtures. We used nitrogen stable isotope ratios to characterize bat diets and adopted a phylogenetically informed approach to investi- gate the physiological changes that accompany evolutionary diet changes in phyllostomids. We found that nitrogen stable isotopes sep- arated plant-eating from animal-eating species. The blood of the latter was enriched in 15N. A recent phylogenetic hypothesis suggests that with the possible exception of carnivory, which may have evolved twice, all diets evolved only once from insectivory. The shift from insectivory to nectarivory and frugivory was accompanied by increased intestinal sucrase and maltase activity, decreased trehalase activity, and reduced relative medullary thickness of kidneys. The shift from insectivory to sanguinivory and carnivory resulted in re- duced trehalase activity. Vampire bats are the only known vertebrates that do not exhibit intestinal maltase activity. We argue that these physiological changes are adaptive responses to evolutionary diet shifts. Key words: Bats, comparative method, diet, digestive and renal function, stable isotopes. Introduction The family Phyllostomidae is a speciose (49 genera and Characterizing animal diets can be difficult. -

Ixodida: Argasidae) En México
Universidad Nacional Autónoma de México Facultad de Estudios Superiores – Zaragoza Diferenciación morfológica y molecular de garrapatas del género Antricola (Ixodida: Argasidae) en México. TESIS Que para obtener el título de: BIÓLOGO Presenta: Jesús Alberto Lugo Aldana Directora de tesis: Dra. María del Carmen Guzmán Cornejo Asesor interno: Dr. David Nahum Espinosa Organista México, D. F. Abril 2013 Facultad de Estudios Superiores Zaragoza, UNAM. *** Laboratorio de Acarología “Anita Hoffman” Facultad de Ciencias, UNAM. Dedicatoria A mi esposa Diana Sánchez y mi pequeña princesa Quetzalli por ser el motivo, para ser mejor día con día. A mi mamá Sara H. Aldana G. y mi papá Jesús G. Lugo C. por enseñarme los valores que hoy me hacen ser una gran persona, además de ser el ejemplo para superarme todos los días. A mi hermana Mitzi Saraí y hermano José Luis por su apoyo incondicional, que además de ser inspiración, lograron alentarme en todo momento para concluir este gran proyecto. A la familia Aldana Sánchez, Aldana Godínez, Del Moral Aldana, Lugo Cid, Lugo Romero, Lugo Maldonado y a la Sra. Irene Cabrera R., por todos sus consejos y apoyo incondicional a lo largo de toda mi carrera. Mil gracias… Agradecimientos o A mi alma mater U.N.A.M., por darme la oportunidad de ser parte de ésta gran institución. o A la Facultad de Estudios Superiores Zaragoza, por brindarme los conocimientos esenciales en toda mi formación como biólogo. o A la Dra. Ma. Del Carmen Guzmán Cornejo, “Meli”, por todo su apoyo incondicional durante la realización de éste trabajo, ya que sin sus consejos, orientación, confianza y amistad no se hubieran alcanzado tantas satisfacciones. -

Biology and Parasites of Pteronotus Gymnonotus from the Caatinga Shrublands of Ceará (Brazil)
THERYA, 2021, Vol. 12(1):131-137 DOI:10.12933/therya-21-1078 ISSN 2007-3364 Biology and parasites of Pteronotus gymnonotus from the Caatinga shrublands of Ceará (Brazil) SHIRLEY SEIXAS PEREIRA DA SILVA1*, PATRÍCIA GONÇALVES GUEDES1, FLÁVIA SILVA SEVERINO1, AND JULIANA CARDOSO DE ALMEIDA2 1 Instituto Resgatando o Verde – IRV. Rua Tirol, 536, sala 609, Freguesia, Rio de Janeiro – RJ, C.P. 22750-009, Brazil. E-mail: [email protected] (SSPDS), [email protected] (PGG), [email protected] (FSS). 2 Instituto Resgatando o Verde – IRV. Rua Tirol, 536, sala 609, Freguesia, Rio de Janeiro – RJ, 22750-009, Brazil. Universidade Iguaçu (UNIG). Av. Abílio Augusto Távora, 2134, Nova Iguaçu – RJ, C.P. 26260-0454, Brazil. Centro Universitário Geraldo di Biase (UGB). Rodovia Benjamim Ielpo, km 11, Parque São José, Barra do Piraí - RJ, C.P. 27101-970, Brazil. E-mail: [email protected]. *Corresponding author Mormoopid bats are distributed from southern United States of America to Brazil and comprise the genera Mormoops and Pteronotus. Although forms of Mormoops in Bahia, Brazil were described for the Quaternary, only some of the extant species of Pteronotus occur in this country, including P. gymnonotus. The species distribution ranges from southeastern México to northeastern Bolivia and central Brazil. This work presents information about food preference, reproduction, and the ectoparasitological fauna of P. gymnonotus in the state of Ceará. Fieldwork took place over ten consecutive days in the rain and dry seasons, in 2000, 2012, 2013, and 2019, on trails within the Serra das Almas Private Natural Heritage Reserve. A total of 14 P. gymnonotus specimens were caught in the three main phytophysiognomies present in the region. -

BATS of the Golfo Dulce Region, Costa Rica
MURCIÉLAGOS de la región del Golfo Dulce, Puntarenas, Costa Rica BATS of the Golfo Dulce Region, Costa Rica 1 Elène Haave-Audet1,2, Gloriana Chaverri3,4, Doris Audet2, Manuel Sánchez1, Andrew Whitworth1 1Osa Conservation, 2University of Alberta, 3Universidad de Costa Rica, 4Smithsonian Tropical Research Institute Photos: Doris Audet (DA), Joxerra Aihartza (JA), Gloriana Chaverri (GC), Sébastien Puechmaille (SP), Manuel Sánchez (MS). Map: Hellen Solís, Universidad de Costa Rica © Elène Haave-Audet [[email protected]] and other authors. Thanks to: Osa Conservation and the Bobolink Foundation. [fieldguides.fieldmuseum.org] [1209] version 1 11/2019 The Golfo Dulce region is comprised of old and secondary growth seasonally wet tropical forest. This guide includes representative species from all families encountered in the lowlands (< 400 masl), where ca. 75 species possibly occur. Species checklist for the region was compiled based on bat captures by the authors and from: Lista y distribución de murciélagos de Costa Rica. Rodríguez & Wilson (1999); The mammals of Central America and Southeast Mexico. Reid (2012). Taxonomy according to Simmons (2005). La región del Golfo Dulce está compuesta de bosque estacionalmente húmedo primario y secundario. Esta guía incluye especies representativas de las familias presentes en las tierras bajas de la región (< de 400 m.s.n.m), donde se puede encontrar c. 75 especies. La lista de especies fue preparada con base en capturas de los autores y desde: Lista y distribución de murciélagos de Costa Rica. Rodríguez -

Quaternary Bat Diversity in the Dominican Republic
AMERICAN MUSEUM NOVITATES Number 3779, 20 pp. June 21, 2013 Quaternary Bat Diversity in the Dominican Republic PAÚL M. VELAZCO,1 HANNAH O’NEILL,2 GREGG F. GUNNELL,3 SIOBHÁN B. COOKE,4 RENATO RIMOLI,5 ALFRED L. ROSENBErgER,1, 6 AND NANCY B. SIMMONS1 ABSTRACT The fossil record of bats is extensive in the Caribbean, but few fossils have previously been reported from the Dominican Republic. In this paper, we describe new collections of fossil bats from two flooded caves in the Dominican Republic, and summarize previous finds from the Island of Hispaniola. The new collections were evaluated in the context of extant and fossil faunas of the Greater Antilles to provide information on the evolution of the bat community of Hispaniola. Eleven species were identified within the new collections, including five mormoopids (Mormoops blainvillei, †Mormoops magna, Pteronotus macleayii, P. parnellii, and P. quadridens), five phyllostomids (Brachy- phylla nana, Monophyllus redmani, Phyllonycteris poeyi, Erophylla bombifrons, and Phyllops falcatus), and one natalid (Chilonatalus micropus). All of these species today inhabitant Hispaniola with the exception of †Mormoops magna, an extinct species previously known only from the Quaternary of Cuba, and Pteronotus macleayii, which is currently known only from extant populations in Cuba and Jamaica, although Quaternary fossils have also been recovered in the Bahamas. Differences between the fossil faunas and those known from the island today suggest that dispersal and extirpa- tion events, perhaps linked to climate change or stochastic events such as hurricanes, may have played roles in structuring the modern fauna of Hispaniola. 1 Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Interações Taxonômicas Entre Parasitos E Morcegos De Alguns Municípios Do Estado De Minas Gerais
UNIVERSIDADE FEDERAL DE MINAS GERAIS INSTITUTO DE CIÊNCIAS BIOLÓGICAS PROGRAMA DE PÓS-GRADUAÇÃO EM PARASITOLOGIA INTERAÇÕES TAXONÔMICAS ENTRE PARASITOS E MORCEGOS DE ALGUNS MUNICÍPIOS DO ESTADO DE MINAS GERAIS. ÉRICA MUNHOZ DE MELLO BELO HORIZONTE ÉRICA MUNHOZ DE MELLO INTERAÇÕES TAXONÔMICAS ENTRE PARASITOS E MORCEGOS DE ALGUNS MUNICÍPIOS DO ESTADO DE MINAS GERAIS. Tese apresentada ao Programa de Pós-Graduação em Parasitologia do Instituto de Ciências Biológicas da Universidade Federal de Minas Gerais, como requisito parcial à obtenção do título de Doutora em Parasitolog ia. Área de concentração: Helmintologia Orientação: Dra. Élida Mara Leite Rabelo/UFMG Co -Orientação: Dr. Reinaldo José da Silva/UNESP BELO HORIZONTE 2017 À minha família e aos meus amigos pelo apoio e compreensão. À todos os meus mestres pelos incentivos e contribuições na minha formação. AGRADECIMENTOS À minha orientadora, Élida Mara Leite Rabelo, que desde sempre me incentivou, confiou na minha capacidade, me deu total liberdade para desenvolver a tese e foi muito participativa ao longo de todo o processo. Muito obrigada por todos os ensinamentos, toda ajuda e todo o apoio de amiga, as vezes de mãe. Te ter como orientadora foi uma honra e eternamente serei grata por isso. Ao meu co-orientador, Reinaldo José da Silva, que mesmo de longe, sempre esteve presente ao longo de todo o doutorado. Muito obrigada pelos ensinamentos, pelo seu esforço em me ajudar ao máximo nas minhas visitas relâmpagos à Botucatu, e pela confiança no meu trabalho. Sempre será um privilégio trabalhar com você. Às bancas da qualificação e da defesa final por todas as sugestões, muito obrigada. -

Bat Diversity in Three Roosts in the Coast Region of Oaxaca, México
Neotropical Biology and Conservation 15(2): 135–152 (2020) doi: 10.3897/neotropical.15.e50136 RESEarcH ARTICLE Bat diversity in three roosts in the Coast region of Oaxaca, México Diversidade de morcegos em três abrigos na região costeira de Oaxaca, México Itandehui Hernández-Aguilar1, Antonio Santos-Moreno1 1 Laboratorio de Ecología Animal. Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Oaxaca. Instituto Politécnico Nacional. Calle Hornos No. 1003, Col. La Noche Buena, Santa Cruz Xoxocotlán, Código Postal 71230, Oaxaca, México Corresponding author: Antonio Santos-Moreno ([email protected]) Academic editor: A. M. Leal-Zanchet | Received 15 January 2020 | Accepted 31 March 2020 | Published 29 May 2020 Citation: Hernández-Aguilar I, Santos-Moreno A (2020) Bat diversity in three roosts in the Coast region of Oaxaca, México. Neotropical Biology and Conservation 15(2): 135–152. https://doi.org/10.3897/neotropical.15.e50136 Abstract In this paper, we analyze the richness, abundance, diversity and trophic guilds in a mine (La Mina) and two caves (El Apanguito and Cerro Huatulco) in the municipalities of Pluma Hidalgo and Santa María Huatulco, in the state of Oaxaca, México, a state with high species richness of bats nationwide. Fieldwork was conducted from July 2016 to June 2017. Using a harp trap, we captured 5,836 bats belonging to 14 species, 10 genera and five families. The greatest species richness was found in Cerro Huatulco (12 species), followed by La Mina (nine species) and El Apanguito (four species). Overall, the most abundant species were Pteronotus fulvus (40.59% of captures) and Pteronotus mesoamericanus (32.01%). -

09 Jose Brites.Indd
Veterinary World, EISSN: 2231-0916 REVIEW ARTICLE Available at www.veterinaryworld.org/Vol.8/March-2015/9.pdf Open Access Tick-borne infections in human and animal population worldwide José Brites-Neto1, Keila Maria Roncato Duarte2 and Thiago Fernandes Martins3 1. Department of Public Health, Americana, São Paulo, Brazil; 2. Department of Genetics and Animal Reproduction, Institute of Animal Science, Nova Odessa, São Paulo, Brazil; 3. Department of Preventive Veterinary Medicine, Faculty of Veterinary Medicine and Animal Sciences, University of São Paulo, São Paulo, Brazil. Corresponding author: José Brites-Neto, e-mail: [email protected], KMRD: [email protected], TFM: [email protected] Received: 14-11-2014, Revised: 20-01-2015, Accepted: 25-01-2015, Published online: 12-03-2015 doi: 10.14202/vetworld.2015.301-315. How to cite this article: Brites-Neto J, Duarte KMR, Martins TF (2015) Tick- borne infections in human and animal population worldwide, Veterinary World 8(3):301-315. Abstract The abundance and activity of ectoparasites and its hosts are affected by various abiotic factors, such as climate and other organisms (predators, pathogens and competitors) presenting thus multiples forms of association (obligate to facultative, permanent to intermittent and superficial to subcutaneous) developed during long co-evolving processes. Ticks are ectoparasites widespread globally and its eco epidemiology are closely related to the environmental conditions. They are obligatory hematophagous ectoparasites and responsible as vectors or reservoirs at the transmission of pathogenic fungi, protozoa, viruses, rickettsia and others bacteria during their feeding process on the hosts. Ticks constitute the second vector group that transmit the major number of pathogens to humans and play a role primary for animals in the process of diseases transmission. -
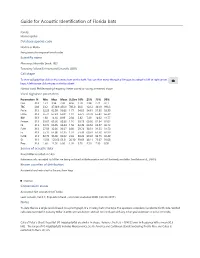
Mormoops Blainvillei Leach, 1821 Taxonomy Follows Simmons and Cirranello (2021) Call Shape
Guide for Acoustic Identification of Florida bats Family: Mormoopidae Database species code Morbla or Moba See glossary for explanation of codes Scientific name Mormoops blainvillei Leach, 1821 Taxonomy follows Simmons and Cirranello (2021) Call shape To view call graphics click on the camera icon on the right. You can then move through all images by using the left or right arrow keys. A left mouse click returns to the fact sheet. Narrow band, FM decreasing frequency. Never paired or having a reverse J shape Vocal signature parameters Parameters N Min Max Mean St.Dev 10% 25% 75% 90% Dur 313 1.51 3.94 2.36 0.56 1.70 1.94 2.71 3.17 TBC 238 33.2 4730.9 456.9 785.0 60.0 122.2 333.9 993.3 Fmin 313 52.29 62.99 56.03 1.77 54.05 54.61 57.35 58.39 Fmax 313 56.74 67.23 65.01 1.47 63.75 64.78 65.84 66.39 BW 313 1.38 14.10 8.99 2.30 5.82 7.49 10.62 11.77 Fmean 313 55.67 63.66 60.86 1.16 59.73 60.36 61.54 62.02 Fk 313 56.74 66.95 64.84 1.50 63.49 64.52 65.57 66.12 FcH1 313 27.59 32.00 30.67 0.80 29.74 30.31 31.25 31.50 Fc 313 55.17 64.00 61.35 1.59 59.48 60.61 62.50 62.99 FcH3 313 82.76 96.00 92.02 2.39 89.22 90.92 93.75 94.49 Sc 313 15.56 125.66 63.51 20.30 39.69 48.11 75.67 91.08 Pmc 313 1.60 14.50 6.00 1.95 3.70 4.50 7.30 8.50 Source of acoustic data Bruce Miller recorded in Cuba Reference calls recorded by Miller are being archived at BioAcoustica and will be freely available.