An Evaluation of Solanum Nigrum and S. Physalifolium Biology And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Morelloid Clade of Solanum L. (Solanaceae) in Argentina: Nomenclatural Changes, Three New Species and an Updated Key to All Taxa
A peer-reviewed open-access journal PhytoKeys 164: 33–66 (2020) Morelloids in Argentina 33 doi: 10.3897/phytokeys.164.54504 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research The Morelloid clade of Solanum L. (Solanaceae) in Argentina: nomenclatural changes, three new species and an updated key to all taxa Sandra Knapp1, Franco Chiarini2, Juan J. Cantero2,3, Gloria E. Barboza2 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 2 Museo Botánico, IMBIV (Instituto Multidisciplinario de Biología Vegetal), Universidad Nacional de Córdoba, Casilla de Correo 495, 5000, Córdoba, Argentina 3 Departamento de Biología Agrícola, Facultad de Agronomía y Ve- terinaria, Universidad Nacional de Rio Cuarto, Ruta Nac. 36, km 601, 5804, Río Cuarto, Córdoba, Argentina Corresponding author: Sandra Knapp ([email protected]) Academic editor: L. Giacomin | Received 20 May 2020 | Accepted 28 August 2020 | Published 21 October 2020 Citation: Knapp S, Chiarini F, Cantero JJ, Barboza GE (2020) The Morelloid clade of Solanum L. (Solanaceae) in Argentina: nomenclatural changes, three new species and an updated key to all taxa. PhytoKeys 164: 33–66. https://doi. org/10.3897/phytokeys.164.54504 Abstract Since the publication of the Solanaceae treatment in “Flora Argentina” in 2013 exploration in the coun- try and resolution of outstanding nomenclatural and circumscription issues has resulted in a number of changes to the species of the Morelloid clade of Solanum L. (Solanaceae) for Argentina. Here we describe three new species: Solanum hunzikeri Chiarini & Cantero, sp. nov., from wet high elevation areas in Argentina (Catamarca, Salta and Tucumán) and Bolivia (Chuquisaca and Tarija), S. -

Solanum Elaeagnifolium Cav. R.J
R.A. Stanton J.W. Heap Solanum elaeagnifolium Cav. R.J. Carter H. Wu Name Lower leaves c. 10 × 4 cm, oblong-lanceolate, distinctly sinuate-undulate, upper leaves smaller, Solanum elaeagnifolium Cav. is commonly known oblong, entire, venation usually prominent in in Australia as silverleaf nightshade. Solanum is dried specimens, base rounded or cuneate, apex from the Latin solamen, ‘solace’ or ‘comfort’, in acute or obtuse; petiole 0.5–2 cm long, with reference to the narcotic effects of some Solanum or without prickles. Inflorescence a few (1–4)- species. The species name, elaeagnifolium, is flowered raceme at first terminal, soon lateral; Latin for ‘leaves like Elaeagnus’, in reference peduncle 0.5–1 cm long; floral rachis 2–3 cm to olive-like shrubs in the family Elaeagnaceae. long; pedicels 1 cm long at anthesis, reflexed ‘Silverleaf’ refers to the silvery appearance of and lengthened to 2–3 cm long in fruit. Calyx the leaves and ‘nightshade’ is derived from the c. 1 cm long at anthesis; tube 5 mm long, more Anglo-Saxon name for nightshades, ‘nihtscada’ or less 5-ribbed by nerves of 5 subulate lobes, (Parsons and Cuthbertson 1992). Other vernacu- whole enlarging in fruit. Corolla 2–3 cm diam- lar names are meloncillo del campo, tomatillo, eter, rotate-stellate, often reflexed, blue, rarely white horsenettle, bullnettle, silver-leaf horsenet- pale blue, white, deep purple, or pinkish. Anthers tle, tomato weed, sand brier, trompillo, melon- 5–8 mm long, slender, tapered towards apex, cillo, revienta caballo, silver-leaf nettle, purple yellow, conspicuous, erect, not coherent; fila- nightshade, white-weed, western horsenettle, ments 3–4 mm long. -
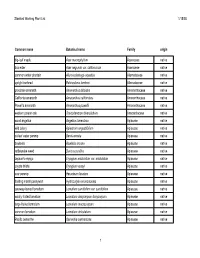
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -

Comparative Biology of Seed Dormancy-Break and Germination in Convolvulaceae (Asterids, Solanales)
University of Kentucky UKnowledge University of Kentucky Doctoral Dissertations Graduate School 2008 COMPARATIVE BIOLOGY OF SEED DORMANCY-BREAK AND GERMINATION IN CONVOLVULACEAE (ASTERIDS, SOLANALES) Kariyawasam Marthinna Gamage Gehan Jayasuriya University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Jayasuriya, Kariyawasam Marthinna Gamage Gehan, "COMPARATIVE BIOLOGY OF SEED DORMANCY- BREAK AND GERMINATION IN CONVOLVULACEAE (ASTERIDS, SOLANALES)" (2008). University of Kentucky Doctoral Dissertations. 639. https://uknowledge.uky.edu/gradschool_diss/639 This Dissertation is brought to you for free and open access by the Graduate School at UKnowledge. It has been accepted for inclusion in University of Kentucky Doctoral Dissertations by an authorized administrator of UKnowledge. For more information, please contact [email protected]. ABSTRACT OF DISSERTATION Kariyawasam Marthinna Gamage Gehan Jayasuriya Graduate School University of Kentucky 2008 COMPARATIVE BIOLOGY OF SEED DORMANCY-BREAK AND GERMINATION IN CONVOLVULACEAE (ASTERIDS, SOLANALES) ABSRACT OF DISSERTATION A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Art and Sciences at the University of Kentucky By Kariyawasam Marthinna Gamage Gehan Jayasuriya Lexington, Kentucky Co-Directors: Dr. Jerry M. Baskin, Professor of Biology Dr. Carol C. Baskin, Professor of Biology and of Plant and Soil Sciences Lexington, Kentucky 2008 Copyright © Gehan Jayasuriya 2008 ABSTRACT OF DISSERTATION COMPARATIVE BIOLOGY OF SEED DORMANCY-BREAK AND GERMINATION IN CONVOLVULACEAE (ASTERIDS, SOLANALES) The biology of seed dormancy and germination of 46 species representing 11 of the 12 tribes in Convolvulaceae were compared in laboratory (mostly), field and greenhouse experiments. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Targeted Weed Control in Potato
TARGETED WEED CONTROL IN POTATO Pamela J.S. Hutchinson Potato Cropping Systems Weed Scientist University of Idaho Aberdeen R&E Center Potato Program of Distinction Potato Program of Distinction What’s up for today? Know your nightshades Witch’s Brews – targeted control UI results Know your nightshades • Hairy nightshade (Solanum physalifolium) is a hard-to- control nightshade weed in potato production fields • Cutleaf (S. triflorum) and Eastern black nightshade (S. ptycanthum) are also in these areas • Black (S. nigrum) and hairy nightshade are known to produce hybrids which can be aggressive Potato Program of Distinction HAIRY NIGHTSHADE Introduced from South America in the 1800’s ▪ First collected in ballast at Nanaimo, Vancouver Island in 1887 Solanum physalifolium (SOLSA) ▪ The weed formerly known as Solanum sarrachoides ▪ Same family as potato (Solanum tuberosum) Problem weed in potatoes ▪ Competition resulting in yield losses, harvest interference; hosts potato pests Potato Program of Distinction HAIRY NIGHTSHADE Annual – 12 to 24 inches • However, hairy nightshade with spreading growth is common Begins germination in early spring and continues germinating throughout the summer • Doesn’t need light to germinate • Germinates under wide temperature range Produces flowers and fruit until the end of the growing season in PNW: • Can produce viable seed as soon as 4 to 5 weeks after flowering and as late as 6 to 7 weeks before a killing frost - A light frost does not kill Potato Program of Distinction HAIRY NIGHTSHADE Hairy nightshade -

Antibacterial and Antioxidant Activities of the Volatile Composition of The
Pharmaceutical Sciences March 2017, 23, 66-71 doi: 10.15171/PS.2017.10 http://journals.tbzmed.ac.ir/PHARM Research Article Antibacterial and Antioxidant Activities of the Volatile Composition of the Flower and Fruit of Solanum sisymbriifolium (Litchi Tomato) Ardalan Pasdaran1,2*, Arsalan Pasdaran3, Nazim Mamedov4 1Department of Pharmacognosy, School of Pharmacy, Research and Development Center of Plants and Medicinal Chemistry, Guilan University of Medical Sciences, Rasht, Iran. 2Medicinal Plants Processing Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. 3Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 4Medicinal Plants Program, Stockbridge School of Agriculture University of Massachusetts at Amherst, USA. A r t i c l e I n f o A B S T R A C T Article History: Background: Solanum sisymbriifolium Lam. is used as traditional remedy in Received: 17 September 2016 South America, recently this plant considered as new edible source. Berries Accepted: 23 January 2017 ePublished: 30 March 2017 and flower of S. sisymbriifolium have a characteristic fragrance. The pleasant fragrance of the S. sisymbriifolium could be considered as a source of food Keywords: additive or preservative. -Gas chromatography–Mass Methods: The essential oils of the flower and fruit of S. sisymbriifolium Lam. spectroscopy -Solanum sisymbriifolium (litchi tomato) were isolated by hydrodistillation method and tested for -Litchi tomato antibacterial and antioxidant potentials also these volatile oils analyzed by the -Essential oils gas chromatography-mass spectrometry (GC-MS) and gas chromatography- -Antibacterial activity flame ionization detection (GC-FID).The antimicrobial activity of the -Antioxidant activity essential oils of fruits and flowers were tested against Staphylococcus aureus using the well diffusion method and their free-radical-scavenging activity were assessed by the 2, 2-diphenyl-picryl-hydrazyl (DPPH) assay. -

Solanum (Solanaceae) in Uganda
Bothalia 25,1: 43-59(1995) Solanum (Solanaceae) in Uganda Z.R. BUKENYA* and J.F. CARASCO** Keywords: food crops, indigenous taxa, key. medicinal plants, ornamentals, Solanum. Solanaceae. Uganda, weeds ABSTRACT Of the 41 species, subspecies and cultivar groups in the genus Solanum L. (Solanaceae) that occur in Uganda, about 30 are indigenous. In Uganda several members of the genus are utilised as food crops while others are put to medicinal and ornamental use. Some members are notorious weeds. A key to the species and descriptions of all Solanum species occurring in Uganda are provided. UITTREKSEL Van die 41 spesies, subspesiesen kultivargroepe indie genus Solanum L. (Solanaceae) wat in Uganda voorkom. is sowat 30 inheems. Verskeie lede van die genus word as voedselgewasse benut. terwyl ander vir geneeskundige en omamentele gebruike aangewend word. Sommige lede is welbekend as onkruide. n Sleutel tot die spesies en beskrvw ings van al die Solanum-spes\cs wat in Uganda voorkom word voorsien. CONTENTS C. Subgenus Leptostemonum (Dunal) Bitter ........ 50 Section Acanthophora Dunal ............................... 51 Introduction............................................................... 44 15. S. mammosum L............................................. 51 Materials and m ethods............................................ 45 16. S. aculeatissimum Jacq................................... 51 Key to species........................................................... 45 Section Aeuleigerum Seithe .................................. 51 Solanum L................................................................. -

Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread
agriculture Review Aphid–Plant–Phytovirus Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread Junior Corneille Fingu-Mabola * and Frédéric Francis Entomologie Fonctionnelle et Évolutive, Terra, Gembloux Agro-Bio Tech, Liège-Université, Passage des Déportés 2, 5030 Gembloux, Belgium; [email protected] * Correspondence: jcfi[email protected] Abstract: Aphids are responsible for the spread of more than half of the known phytovirus species. Virus transmission within the plant–aphid–phytovirus pathosystem depends on vector mobility which allows the aphid to reach its host plant and on vector efficiency in terms of ability to transmit phytoviruses. However, several other factors can influence the phytoviruses transmission process and have significant epidemiological consequences. In this review, we aimed to analyse the aphid behaviours and influencing factors affecting phytovirus spread. We discussed the impact of vector host-seeking and dispersal behaviours mostly involved in aphid-born phytovirus spread but also the effect of feeding behaviours and life history traits involved in plant–aphid–phytovirus relationships on vector performances. We also noted that these behaviours are influenced by factors inherent to the interactions between pathosystem components (mode of transmission of phytoviruses, vector efficiency, plant resistance, ... ) and several biological, biochemical, chemical or physical factors related to the environment of these pathosystem components, most of them being manipulated as means to control vector-borne diseases in the crop fields. Citation: Fingu-Mabola, J.C.; Francis, Keywords: host selection; plant–aphid–virus pathosystem; vector activity; vector-born virus; F. Aphid–Plant–Phytovirus vectorial transmission efficiency Pathosystems: Influencing Factors from Vector Behaviour to Virus Spread. Agriculture 2021, 11, 502. -

New Jersey Strategic Management Plan for Invasive Species
New Jersey Strategic Management Plan for Invasive Species The Recommendations of the New Jersey Invasive Species Council to Governor Jon S. Corzine Pursuant to New Jersey Executive Order #97 Vision Statement: “To reduce the impacts of invasive species on New Jersey’s biodiversity, natural resources, agricultural resources and human health through prevention, control and restoration, and to prevent new invasive species from becoming established.” Prepared by Michael Van Clef, Ph.D. Ecological Solutions LLC 9 Warren Lane Great Meadows, New Jersey 07838 908-637-8003 908-528-6674 [email protected] The first draft of this plan was produced by the author, under contract with the New Jersey Invasive Species Council, in February 2007. Two subsequent drafts were prepared by the author based on direction provided by the Council. The final plan was approved by the Council in August 2009 following revisions by staff of the Department of Environmental Protection. Cover Photos: Top row left: Gypsy Moth (Lymantria dispar); Photo by NJ Department of Agriculture Top row center: Multiflora Rose (Rosa multiflora); Photo by Leslie J. Mehrhoff, University of Connecticut, Bugwood.org Top row right: Japanese Honeysuckle (Lonicera japonica); Photo by Troy Evans, Eastern Kentucky University, Bugwood.org Middle row left: Mile-a-Minute (Polygonum perfoliatum); Photo by Jil M. Swearingen, USDI, National Park Service, Bugwood.org Middle row center: Canadian Thistle (Cirsium arvense); Photo by Steve Dewey, Utah State University, Bugwood.org Middle row right: Asian -

Annotated Checklist of the Vascular Plant Flora of Grand Canyon-Parashant National Monument Phase II Report
Annotated Checklist of the Vascular Plant Flora of Grand Canyon-Parashant National Monument Phase II Report By Dr. Terri Hildebrand Southern Utah University, Cedar City, UT and Dr. Walter Fertig Moenave Botanical Consulting, Kanab, UT Colorado Plateau Cooperative Ecosystems Studies Unit Agreement # H1200-09-0005 1 May 2012 Prepared for Grand Canyon-Parashant National Monument Southern Utah University National Park Service Mojave Network TABLE OF CONTENTS Page # Introduction . 4 Study Area . 6 History and Setting . 6 Geology and Associated Ecoregions . 6 Soils and Climate . 7 Vegetation . 10 Previous Botanical Studies . 11 Methods . 17 Results . 21 Discussion . 28 Conclusions . 32 Acknowledgments . 33 Literature Cited . 34 Figures Figure 1. Location of Grand Canyon-Parashant National Monument in northern Arizona . 5 Figure 2. Ecoregions and 2010-2011 collection sites in Grand Canyon-Parashant National Monument in northern Arizona . 8 Figure 3. Soil types and 2010-2011 collection sites in Grand Canyon-Parashant National Monument in northern Arizona . 9 Figure 4. Increase in the number of plant taxa confirmed as present in Grand Canyon- Parashant National Monument by decade, 1900-2011 . 13 Figure 5. Southern Utah University students enrolled in the 2010 Plant Anatomy and Diversity course that collected during the 30 August 2010 experiential learning event . 18 Figure 6. 2010-2011 collection sites and transportation routes in Grand Canyon-Parashant National Monument in northern Arizona . 22 2 TABLE OF CONTENTS Page # Tables Table 1. Chronology of plant-collecting efforts at Grand Canyon-Parashant National Monument . 14 Table 2. Data fields in the annotated checklist of the flora of Grand Canyon-Parashant National Monument (Appendices A, B, C, and D) . -
Dichotomous Keys to the Species of Solanum L
A peer-reviewed open-access journal PhytoKeysDichotomous 127: 39–76 (2019) keys to the species of Solanum L. (Solanaceae) in continental Africa... 39 doi: 10.3897/phytokeys.127.34326 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Dichotomous keys to the species of Solanum L. (Solanaceae) in continental Africa, Madagascar (incl. the Indian Ocean islands), Macaronesia and the Cape Verde Islands Sandra Knapp1, Maria S. Vorontsova2, Tiina Särkinen3 1 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK 2 Compa- rative Plant and Fungal Biology Department, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AE, UK 3 Royal Botanic Garden Edinburgh, 20A Inverleith Row, Edinburgh EH3 5LR, UK Corresponding author: Sandra Knapp ([email protected]) Academic editor: Leandro Giacomin | Received 9 March 2019 | Accepted 5 June 2019 | Published 19 July 2019 Citation: Knapp S, Vorontsova MS, Särkinen T (2019) Dichotomous keys to the species of Solanum L. (Solanaceae) in continental Africa, Madagascar (incl. the Indian Ocean islands), Macaronesia and the Cape Verde Islands. PhytoKeys 127: 39–76. https://doi.org/10.3897/phytokeys.127.34326 Abstract Solanum L. (Solanaceae) is one of the largest genera of angiosperms and presents difficulties in identifica- tion due to lack of regional keys to all groups. Here we provide keys to all 135 species of Solanum native and naturalised in Africa (as defined by World Geographical Scheme for Recording Plant Distributions): continental Africa, Madagascar (incl. the Indian Ocean islands of Mauritius, La Réunion, the Comoros and the Seychelles), Macaronesia and the Cape Verde Islands. Some of these have previously been pub- lished in the context of monographic works, but here we include all taxa.