Macroalgal Morphology Mediates Particle Capture by the Corallimorpharian Corynactis Californica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Getative Tissues; the GDBH Predicts Metabolic Costs Associated with Them
J. Phycol. 35, 483±492 (1999) PHLOROTANNIN ALLOCATION AMONG TISSUES OF NORTHEASTERN PACIFIC KELPS AND ROCKWEEDS1 Kathryn L. Van Alstyne2 Department of Zoology, Oregon State University, Corvallis, Oregon 97331 James J. McCarthy III, Cynthia L. Hustead, and Laura J. Kearns Department of Biology, Kenyon College, Gambier, Ohio 43022 Optimal defense theory (ODT) predicts antiher- Abbreviations: DM, dry mass; GDBH, growth±dif- bivore defensive compounds will be allocated so ferentiation balance hypothesis; ODT, optimal de- that the most valuable or most susceptible tissues fense theory will be best defended. The growth±differentiation balance hypothesis (GDBH) predicts that defense al- location will be a result of trade-offs between growth Plants allocate materials and energy among criti- and defense. Thus, these two theories predict op- cal functions such as maintenance, growth, repro- posite allocation patterns with respect to ``valuable,'' duction, and defense (Bazazz and Grace 1997 and actively growing meristematic and reproductive tis- citations therein). It is widely assumed the total sues. ODT predicts that meristems and reproductive amount of resources available for these functions is tissues should have higher defense levels than non- limited and all of these functions have signi®cant meristematic vegetative tissues; the GDBH predicts metabolic costs associated with them. Consequently, the defense levels of meristems and reproductive over evolutionary time there should be selection for tissues will be lower than vegetative tissues. We ex- individuals to distribute resources among functions amined allocation patterns of phlorotannins in 21 in ways that maximize overall ®tness, assuming that species of kelps (Order Laminariales) and rock- allocation strategies are not limited by physiological weeds (Order Fucales) from nine sites on the west or other constraints. -

Factors Affecting the Abundance of Paracyathus Stearns!! on Subtidal Rock Walls
MLML / M8~RllIBR;jRY 8272 MOSS LANDING RD. MOSS LANDING, CA 95039 FACTORS AFFECTING THE ABUNDANCE OF PARACYATHUS STEARNS!! ON SUBTIDAL ROCK WALLS by Mark Pranger A thesis submitted in partial fulfillment ofthe requirements for the degree of Master ofScience in Marine Science in the School ofNatural Sciences California State University, Fresno August 1999 ACKNOWLEDGMENTS I would like to thank the members of my graduate advisory committee for their advice and guidance during this process. Dr. James Nybakken for his interest and education in invertebrate biology and for allowing me to help in the teaching of others. For the insights and humor of Dr. Mike Foster, that helped improve this study and my education. For the help of Dr. Stephen Ervin who in the last hours help me through all CSU Fresno's paper work and deadlines. I am grateful to the staff at the Pollution Studies Lab at Granite Canyon for their help during experiments and for allowing me the use of their working space. I am indebted to the staff at Moss Landing Marine Labs that kept all the equipment working and operational, and to the Dr. Earl and Ethel Myers Oceanographic and Marine Biology Trust for helping to fund this project. Most of all, thanks goes to the many students at Moss Landing. Without their help this study would not have been completed. Special thanks goes to; Mat Edwards for being a faithful dive buddy, to Torno Eguchi, Michelle White, Bryn Phillips, Cassandra Roberts, Michelle Lander, and Lara Lovera for their help on statistics and editing of early drafts, and especially to Michele Jacobi for her help in all aspects of this study and her constant encouragement for me to finish. -

The Diet and Predator-Prey Relationships of the Sea Star Pycnopodia Helianthoides (Brandt) from a Central California Kelp Forest
THE DIET AND PREDATOR-PREY RELATIONSHIPS OF THE SEA STAR PYCNOPODIA HELIANTHOIDES (BRANDT) FROM A CENTRAL CALIFORNIA KELP FOREST A Thesis Presented to The Faculty of Moss Landing Marine Laboratories San Jose State University In Partial Fulfillment of the Requirements for the Degree Master of Arts by Timothy John Herrlinger December 1983 TABLE OF CONTENTS Acknowledgments iv Abstract vi List of Tables viii List of Figures ix INTRODUCTION 1 MATERIALS AND METHODS Site Description 4 Diet 5 Prey Densities and Defensive Responses 8 Prey-Size Selection 9 Prey Handling Times 9 Prey Adhesion 9 Tethering of Calliostoma ligatum 10 Microhabitat Distribution of Prey 12 OBSERVATIONS AND RESULTS Diet 14 Prey Densities 16 Prey Defensive Responses 17 Prey-Size Selection 18 Prey Handling Times 18 Prey Adhesion 19 Tethering of Calliostoma ligatum 19 Microhabitat Distribution of Prey 20 DISCUSSION Diet 21 Prey Densities 24 Prey Defensive Responses 25 Prey-Size Selection 27 Prey Handling Times 27 Prey Adhesion 28 Tethering of Calliostoma ligatum and Prey Refugia 29 Microhabitat Distribution of Prey 32 Chemoreception vs. a Chemotactile Response 36 Foraging Strategy 38 LITERATURE CITED 41 TABLES 48 FIGURES 56 iii ACKNOWLEDGMENTS My span at Moss Landing Marine Laboratories has been a wonderful experience. So many people have contributed in one way or another to the outcome. My diving buddies perse- vered through a lot and I cherish our camaraderie: Todd Anderson, Joel Thompson, Allan Fukuyama, Val Breda, John Heine, Mike Denega, Bruce Welden, Becky Herrlinger, Al Solonsky, Ellen Faurot, Gilbert Van Dykhuizen, Ralph Larson, Guy Hoelzer, Mickey Singer, and Jerry Kashiwada. Kevin Lohman and Richard Reaves spent many hours repairing com puter programs for me. -

Variation in Blade Morphology of the Kelp Eisenia Arborea: Incipient Speciation Due to Local Water Motion?
MARINE ECOLOGY PROGRESS SERIES Vol. 282: 115–128, 2004 Published November 16 Mar Ecol Prog Ser Variation in blade morphology of the kelp Eisenia arborea: incipient speciation due to local water motion? Loretta M. Roberson1, 3,* James A. Coyer2 1Hopkins Marine Station, Stanford University, Pacific Grove, California 93950, USA 2Department of Marine Biology, University of Groningen, Kerklaan 30, PO Box 14, 9750 AA Haren, The Netherlands 3Present address: Institute of Neurobiology and Department of Biology, University of Puerto Rico, PO Box 23360, San Juan, Puerto Rico 00931-3360, USA ABSTRACT: The southern sea palm kelp Eisenia arborea produces wide, bullate (bumpy) blades in low-flow areas, whereas in adjacent high-flow areas blades are flat and narrow. Here we determine if morphological differences in these 2 closely associated populations are correlated with physical factors in the environment, and whether this response is a genetically fixed or plastic trait. Both phe- notypes were subjected to morphometric analysis, field and laboratory transplant experiments, and genetic analysis (M13 DNA fingerprinting). Physical variables (water motion, temperature, and light) were monitored simultaneously in the field. Results indicate that the 2 populations, separated by <10m, are genetically distinct and transplants in the field and lab remained similar to individuals from the original collection location, regardless of the imposed flow environment. Water motion var- ied significantly between the 2 populations and was the single physical variable that correlated with morphology. Based on bump heights and water velocities in the field, bullate blades could increase turbulent transport of nutrients 4-fold that of flat blades. The thicker blades and large holdfasts of the flat morph may impart higher survivorship under high-flow conditions. -

Kelp Forest Monitoring Handbook — Volume 1: Sampling Protocol
KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL CHANNEL ISLANDS NATIONAL PARK KELP FOREST MONITORING HANDBOOK VOLUME 1: SAMPLING PROTOCOL Channel Islands National Park Gary E. Davis David J. Kushner Jennifer M. Mondragon Jeff E. Mondragon Derek Lerma Daniel V. Richards National Park Service Channel Islands National Park 1901 Spinnaker Drive Ventura, California 93001 November 1997 TABLE OF CONTENTS INTRODUCTION .....................................................................................................1 MONITORING DESIGN CONSIDERATIONS ......................................................... Species Selection ...........................................................................................2 Site Selection .................................................................................................3 Sampling Technique Selection .......................................................................3 SAMPLING METHOD PROTOCOL......................................................................... General Information .......................................................................................8 1 m Quadrats ..................................................................................................9 5 m Quadrats ..................................................................................................11 Band Transects ...............................................................................................13 Random Point Contacts ..................................................................................15 -
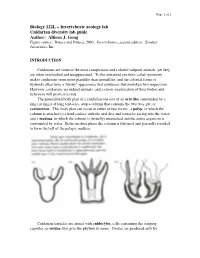
Cnidarians Are Some of the Most Conspicuous and Colorful Tidepool Animals, Yet They Are Often Overlooked and Unappreciated
Page 1 of 1 Biology 122L – Invertebrate zoology lab Cnidarian diversity lab guide Author: Allison J. Gong Figure source: Brusca and Brusca, 2003. Invertebrates, second edition. Sinauer Associates, Inc. INTRODUCTION Cnidarians are some of the most conspicuous and colorful tidepool animals, yet they are often overlooked and unappreciated. To the untrained eye their radial symmetry makes cnidarians seem more plantlike than animallike, and the colonial forms of hydroids often have a "bushy" appearance that reinforces that mistaken first impression. However, cnidarians are indeed animals, and a closer examination of their bodies and behaviors will prove it to you. The generalized body plan of a cnidarian consists of an oral disc surrounded by a ring (or rings) of long tentacles, atop a column that contains the two-way gut, or coelenteron. This body plan can occur in either of two forms: a polyp, in which the column is attached to a hard surface with the oral disc and tentacles facing into the water; and a medusa, in which the column is (usually) unattached and the entire organism is surrounded by water. In the medusa phase the column is flattened and generally rounded to form the bell of the pelagic medusa. Cnidarian tentacles are armed with cnidocytes, cells containing the stinging capsules, or cnidae, that give the phylum its name. Cnidae are produced only by Page 2 of 2 cnidarians, although they can occasionally be found in the cerata of nudibranch molluscs that feed on cnidarians: through a process that is not understood, some nudibranchs are able to ingest cnidae from their prey and sequester them, unfired, in their cerata for defense against their own predators. -

Spatial Distribution and the Effects of Competition on Some Temperate Scleractinia and Corallimorpharia
MARINE ECOLOGY PROGRESS SERIES Vol. 70: 39-48, 1991 Published February 14 Mar. Ecol. Prog. Ser. ~ Spatial distribution and the effects of competition on some temperate Scleractinia and Corallimorpharia Nanette E. Chadwick* Department of Zoology. University of California, Berkeley. California 94720. USA ABSTRACT: The impact of interference competition on coral community structure is poorly understood. On subtidal rocks in the northeastern Pacific, members of 3 scleractinian coral species (Astrangia lajollaensis, Balanophyllia elegans, Paracyathus stearnsii) and 1 corallimorpharian (Corynactls califor- nica) were examined to determine whether competition exerts substantial influence over the~rabund- ance and distributional patterns. These anthozoans occupy > 50 % cover on hard substrata, and exhibit characteristic patterns of spatial distribution, with vertical zonation and segregation among some species. They interact in an interspecific dominance hierarchy that lacks reversals, and is linear and consistent under laboratory and field conditions. Experiments demonstrated that a competitive domin- ant, C. californica, influences the abundance and population structure of a subordinate. B. elegans, by (1)reducing sexual reproductive output, (2) increasing larval mortality, (3)altering recruitment patterns. Field cross-transplants revealed that the dominant also affects vertical zonation of a competitive intermediate, A. lajollaensis, by lulling polyps that occur near the tops of subtidal rocks. It is concluded that between-species competition, -

The Effect of Wave Exposure on the Morphology of Ecklonia Radiata
Aquatic Botany 83 (2005) 61–70 www.elsevier.com/locate/aquabot The effect of wave exposure on the morphology of Ecklonia radiata Thomas Wernberg a,*, Mads S. Thomsen b a School of Plant Biology, Botany Building MO90, University of Western Australia, Crawley, 6009 WA, Australia b Department of Applied Sciences, Auckland University of Technology, Auckland, New Zealand Received 3 August 2004; received in revised form 11 April 2005; accepted 31 May 2005 Abstract This study examined the consistency of the effect of wave exposure on the morphology of Ecklonia radiata, a small kelp, across a broad geographic range (>1100 km). Fifteen morphological characters were measured on individuals from sites of low (2.5 Æ 0.8 S.E.) and high (12.2 Æ 2.0 S.E.) wave exposure (Baardseth’s index) within six locations in southwestern Australia. With the exception of lateral width (P = 0.0001), none of the morphological characters were consistently statistically different (P > 0.06) between high and low wave exposure and correlations with wave exposure were generally weak (r < 0.60). While 12 of the 15 characters were statistically different (P < 0.008) between sites of different exposure within at least one location, the direction of difference (significant or not) was opposite between some locations for all but one character (lateral width). ANOSIM was not able to separate thalli from high and low exposure when all locations were pooled (Clarke’s R = 0.127), but within each location most sites showed better separation (0.126 < Clarke’s R < 0.829). Despite the lack of statistical differences, trends suggested that E. -

Seaweed and Seagrasses Inventory of Laguna De San Ignacio, BCS
UNIVERSIDAD AUTÓNOMA DE BAJA CALIFORNIA SUR ÁREA DE CONOCIMIENTO DE CIENCIAS DEL MAR DEPARTAMENTO ACADÉMICO DE BIOLOGÍA MARINA PROGRAMA DE INVESTIGACIÓN EN BOTÁNICA MARINA Seaweed and seagrasses inventory of Laguna de San Ignacio, BCS. Dr. Rafael Riosmena-Rodríguez y Dr. Juan Manuel López Vivas Programa de Investigación en Botánica Marina, Departamento de Biología Marina, Universidad Autónoma de Baja California Sur, Apartado postal 19-B, km. 5.5 carretera al Sur, La Paz B.C.S. 23080 México. Tel. 52-612-1238800 ext. 4140; Fax. 52-612-12800880; Email: [email protected]. Participants: Dr. Jorge Manuel López-Calderón, Dr. Carlos Sánchez Ortiz, Dr. Gerardo González Barba, Dr. Sung Min Boo, Dra. Kyung Min Lee, Hidrobiol. Carmen Mendez Trejo, M. en C. Nestor Manuel Ruíz Robinson, Pas Biol. Mar. Tania Cota. Periodo de reporte: Marzo del 2013 a Junio del 2014. Abstract: The present report presents the surveys of marine flora 2013 – 2014 in the San Ignacio Lagoon of the, representing the 50% of planned visits and in where we were able to identifying 19 species of macroalgae to the area plus 2 Seagrass traditionally cited. The analysis of the number of species / distribution of macroalgae and seagrass is in progress using an intense review of literature who will be concluded using the last field trip information in May-June 2014. During the last two years we have not been able to find large abundances of species of microalgae as were described since 2006 and the floristic lists developed in the 90's. This added with the presence to increase both coverage and biomass of invasive species which makes a real threat to consider. -

Turf Assemblage of a Macrocystis Kelp Forest: Experiments On
AN ABSTRACT OF THE THESIS OF A. Keith Miles for the degree of Doctor of Philosophy in Wildlife Science presented on June 16. 1986. Title: A m Macrosti t° Ex nmen n and Herbivory. Redacted for privacy Abstract approved: E. Charles Meslow Early and later successional stages of the assemblage of turf algae and sessile animals of a Macrocystis kelp forest were studied off San Nicolas Island, California from 1980 through 1981, and 1983 through 1984. Kelps were manipulated to determine if differences in illumination could account for dominance by turf algae or sessile animals. Caging and algal-removal experiments were conducted to determine if the effects of herbivory and competition could account for the low or variable recruitment and abundance of foliose turf algae. Plots were covered for different time intervals up to 1 year to determine if survival of overgrowth could explain the prevalence of competitively subordinate crustose coralline algae. Increased cover and recruitment of turf algae and decreased cover and low recruitment of sessile animals were correlated with the removal of canopy. In the presence of canopy, existing cover of crustose algae and sessile animalschanged little over time; sessile animals recruited at significantly higher levels thanalgae on cleared plots. Patiria (a common invertebrate grazer) removed certain ephemeral algae and appeared to slow recruitment by other algae, but had little effect on mature turf algae. These effects appeared dependent on a high density of Patiria. Algal recruitment on caged and open, near-bare plots was negligible, but sessile animals recruited heavily to these plots. These results were correlated with low illumination caused by a dense and persistent surface canopy of Macrocystis. -

A Comprehensive Kelp Phylogeny Sheds Light on the Evolution of an T Ecosystem ⁎ Samuel Starkoa,B,C, , Marybel Soto Gomeza, Hayley Darbya, Kyle W
Molecular Phylogenetics and Evolution 136 (2019) 138–150 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A comprehensive kelp phylogeny sheds light on the evolution of an T ecosystem ⁎ Samuel Starkoa,b,c, , Marybel Soto Gomeza, Hayley Darbya, Kyle W. Demesd, Hiroshi Kawaie, Norishige Yotsukuraf, Sandra C. Lindstroma, Patrick J. Keelinga,d, Sean W. Grahama, Patrick T. Martonea,b,c a Department of Botany & Biodiversity Research Centre, The University of British Columbia, 6270 University Blvd., Vancouver V6T 1Z4, Canada b Bamfield Marine Sciences Centre, 100 Pachena Rd., Bamfield V0R 1B0, Canada c Hakai Institute, Heriot Bay, Quadra Island, Canada d Department of Zoology, The University of British Columbia, 6270 University Blvd., Vancouver V6T 1Z4, Canada e Department of Biology, Kobe University, Rokkodaicho 657-8501, Japan f Field Science Center for Northern Biosphere, Hokkaido University, Sapporo 060-0809, Japan ARTICLE INFO ABSTRACT Keywords: Reconstructing phylogenetic topologies and divergence times is essential for inferring the timing of radiations, Adaptive radiation the appearance of adaptations, and the historical biogeography of key lineages. In temperate marine ecosystems, Speciation kelps (Laminariales) drive productivity and form essential habitat but an incomplete understanding of their Kelp phylogeny has limited our ability to infer their evolutionary origins and the spatial and temporal patterns of their Laminariales diversification. Here, we -

Spectral Diversity of Fluorescent Proteins from the Anthozoan Corynactis Californica
Mar Biotechnol (2008) 10:328–342 DOI 10.1007/s10126-007-9072-7 ORIGINAL ARTICLE Spectral Diversity of Fluorescent Proteins from the Anthozoan Corynactis californica Christine E. Schnitzler & Robert J. Keenan & Robert McCord & Artur Matysik & Lynne M. Christianson & Steven H. D. Haddock Received: 7 September 2007 /Accepted: 19 November 2007 /Published online: 11 March 2008 # Springer Science + Business Media, LLC 2007 Abstract Color morphs of the temperate, nonsymbiotic three to four distinct genetic loci that code for these colors, corallimorpharian Corynactis californica show variation in and one morph contains at least five loci. These genes pigment pattern and coloring. We collected seven distinct encode a subfamily of new GFP-like proteins, which color morphs of C. californica from subtidal locations in fluoresce across the visible spectrum from green to red, Monterey Bay, California, and found that tissue– and color– while sharing between 75% to 89% pairwise amino-acid morph-specific expression of at least six different genes is identity. Biophysical characterization reveals interesting responsible for this variation. Each morph contains at least spectral properties, including a bright yellow protein, an orange protein, and a red protein exhibiting a “fluorescent timer” phenotype. Phylogenetic analysis indicates that the Christine E. Schnitzler and Robert J. Keenan contributed equally to FP genes from this species evolved together but that this work. diversification of anthozoan fluorescent proteins has taken Data deposition footnote: