Cavity Characteristics, but Not Habitat, Influence Nest Survival Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract Book
Welcome to the Ornithological Congress of the Americas! Puerto Iguazú, Misiones, Argentina, from 8–11 August, 2017 Puerto Iguazú is located in the heart of the interior Atlantic Forest and is the portal to the Iguazú Falls, one of the world’s Seven Natural Wonders and a UNESCO World Heritage Site. The area surrounding Puerto Iguazú, the province of Misiones and neighboring regions of Paraguay and Brazil offers many scenic attractions and natural areas such as Iguazú National Park, and provides unique opportunities for birdwatching. Over 500 species have been recorded, including many Atlantic Forest endemics like the Blue Manakin (Chiroxiphia caudata), the emblem of our congress. This is the first meeting collaboratively organized by the Association of Field Ornithologists, Sociedade Brasileira de Ornitologia and Aves Argentinas, and promises to be an outstanding professional experience for both students and researchers. The congress will feature workshops, symposia, over 400 scientific presentations, 7 internationally renowned plenary speakers, and a celebration of 100 years of Aves Argentinas! Enjoy the book of abstracts! ORGANIZING COMMITTEE CHAIR: Valentina Ferretti, Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA- CONICET) and Association of Field Ornithologists (AFO) Andrés Bosso, Administración de Parques Nacionales (Ministerio de Ambiente y Desarrollo Sustentable) Reed Bowman, Archbold Biological Station and Association of Field Ornithologists (AFO) Gustavo Sebastián Cabanne, División Ornitología, Museo Argentino -

Birding the Atlantic Rainforest, South-East Brazil Itororo Lodge and Regua 11Th – 20Th March 2018
BIRDING THE ATLANTIC RAINFOREST, SOUTH-EAST BRAZIL ITORORO LODGE AND REGUA 11TH – 20TH MARCH 2018 White-barred Piculet (©Andy Foster) Guided and report compiled by Andy Foster www.serradostucanos.com.br Sunday 11th March The following 10 day tour was a private trip for a group of 4 friends. We all flew in from the UK on a BA flight landing the night of the 10th and stayed in the Linx Hotel located close to the International airport in Rio de Janeiro. We met up for breakfast at 07.00 and by 08.00 our driver had arrived to take us for the 2.5 hour drive to Itororo Lodge where we were to spend our first 6 nights birding the higher elevations of the Serra do Mar Mountains. On the journey up we saw Magnificent Frigatebird, Cocoi Heron, Great White Egret, Black-crowned Night Heron, Neotropic Cormorant and Roadside Hawk. By 10.30 we had arrived at the lodge and were greeted by Bettina and Rainer who would be our hosts for the next week. The feeders were busy at the lodge and we were soon picking up new species including Azure-shouldered Tanager, Brassy-breasted Tanager, Black-goggled Tanager, Sayaca Tanager, Ruby- crowned Tanager, Golden-chevroned Tanager, Magpie Tanager, Burnished-buff Tanager, Plain Parakeet, Maroon-bellied Parakeet, Rufous-bellied Thrush, Green-winged Saltator, Pale-breasted Thrush, Violet- capped Woodnymph, Black Jacobin, Scale-throated Hermit, Sombre Hummingbird, Brazilian Ruby and White-throated Hummingbird…. not bad for the first 30 minutes! We spent the last hour or so before lunch getting to grips with the feeder birds, we also picked up brief but good views of a Black-Hawk Eagle as it flew through the lodge gardens. -

Birds of Brazil
BIRDS OF BRAZIL - MP3 SOUND COLLECTION version 2.0 List of recordings 0001 1 Greater Rhea 1 Song 0:17 Rhea americana (20/7/2005, Chapada dos Guimaraes, Mato Grosso, Brazil, 15.20S,55.50W) © Peter Boesman 0006 1 Gray Tinamou 1 Song 0:43 Tinamus tao (15/8/2007 18:30h, Nirgua area, San Felipe, Venezuela, 10.15N,68.30W) © Peter Boesman 0006 2 Gray Tinamou 2 Song 0:24 Tinamus tao (2/1/2008 17:15h, Tarapoto tunnel road, San Martín, Peru, 06.25S,76.15W) © Peter Boesman 0006 3 Gray Tinamou 3 Whistle 0:09 Tinamus tao (15/8/2007 18:30h, Nirgua area, San Felipe, Venezuela, 10.15N,68.30W) © Peter Boesman 0007 1 Solitary Tinamou 1 Song () 0:05 Tinamus solitarius (11/8/2004 08:00h, Serra da Graciosa, Paraná, Brazil, 25.20S,48.55W) © Peter Boesman. 0009 1 Great Tinamou 1 Song 1:31 Tinamus major (3/1/2008 18:45h, Morro de Calzada, San Martín, Peru, 06.00S,77.05W) © Peter Boesman 0009 2 Great Tinamou 2 Song 0:31 Tinamus major (28/7/2009 18:00h, Pantiacolla Lodge, Madre de Dios, Peru, 12.39S,71.14W) © Peter Boesman 0009 3 Great Tinamou 3 Song 0:27 Tinamus major (26/7/2009 17:00h, Pantiacolla Lodge, Madre de Dios, Peru, 12.39S,71.14W) © Peter Boesman 0009 4 Great Tinamou 4 Song 0:46 Tinamus major (22nd July 2010 17h00, ACTS Explornapo, Loreto, Peru, 120 m. 3°10' S, 72°55' W). (Background: Thrush-like Antpitta, Elegant Woodcreeper). © Peter Boesman. 0009 5 Great Tinamou 5 Call 0:11 Tinamus major (17/7/2006 17:30h, Iracema falls, Presidente Figueiredo, Amazonas, Brazil, 02.00S,60.00W) © Peter Boesman. -

Rochely Santos Morandini
Rochely Santos Morandini Diversidade funcional das aves do Cerrado com simulações da perda de fisionomias campestres e de espécies ameaçadas: implicações para a conservação. (VERSÃO CORRIGIDA – versão original disponível na Biblioteca do IB-USP e na Biblioteca Digital de Teses e Dissertações (BDTD) da USP) Functional Diversity of Cerrado birds with a simulation of the loss of open areas and endangered species: implications for conservation. São Paulo 2013 Rochely Santos Morandini Diversidade funcional das aves do Cerrado com simulações da perda de fisionomias campestres e de espécies ameaçadas: implicações para a conservação. Functional Diversity of Cerrado birds with a simulation of the loss of open areas and endangered species: implications for conservation. Dissertação apresentada ao Instituto de Biociências da Universidade de São Paulo para a obtenção do Título de Mestre em Ciências, na Área de Ecologia. Orientador: Prof. Dr. José Carlos Motta Junior. São Paulo 2013 Morandini, Rochely Santos Diversidade funcional das aves do Cerrado com simulações da perda de fisionomias campestres e de espécies ameaçadas: implicações para conservação. 112 páginas Dissertação (Mestrado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Ecologia. 1. Aves 2. Cerrado 3. Diversidade Funcional I. Universidade de São Paulo. Instituto de Biociências. Departamento de Ecologia Comitê de Acompanhamento: Luís Fábio Silveira Marco Antônio P. L. Batalha Comissão Julgadora: ________________________ ________________________ Prof(a). Dr. Marco Ant ônio Prof(a). Dr. Sergio Tadeu Meirelles Monteiro Granzinolli ____________________________________ Orientador: Prof. Dr. José Carlos Motta Junior Dedicatória A melhor lembrança que tenho da infância são as paisagens de minha terra natal. Dedico este estudo ao Cerrado, com seus troncos retorcidos, seu amanhecer avermelhado, paisagens onde habitam aves tão encantadoras que me tonteiam. -

Southeast Brazil: Atlantic Rainforest and Savanna, Oct-Nov 2016
Tropical Birding Trip Report Southeast Brazil: Atlantic Rainforest and Savanna, Oct-Nov 2016 SOUTHEAST BRAZIL: Atlantic Rainforest and Savanna October 20th – November 8th, 2016 TOUR LEADER: Nick Athanas Report and photos by Nick Athanas Helmeted Woodpecker - one of our most memorable sightings of the tour It had been a couple of years since I last guided this tour, and I had forgotten how much fun it could be. We covered a lot of ground and visited a great series of parks, lodges, and reserves, racking up a respectable group list of 459 bird species seen as well as some nice mammals. There was a lot of rain in the area, but we had to consider ourselves fortunate that the rainiest days seemed to coincide with our long travel days, so it really didn’t cost us too much in the way of birds. My personal trip favorite sighting was our amazing and prolonged encounter with a rare Helmeted Woodpecker! Others of note included extreme close-ups of Spot-winged Wood-Quail, a surprise Sungrebe, multiple White-necked Hawks, Long-trained Nightjar, 31 species of antbirds, scope views of Variegated Antpitta, a point-blank Spotted Bamboowren, tons of colorful hummers and tanagers, TWO Maned Wolves at the same time, and Giant Anteater. This report is a bit light on text and a bit heavy of photos, mainly due to my insane schedule lately where I have hardly had any time at home, but all photos are from the tour. www.tropicalbirding.com +1-409-515-9110 [email protected] Tropical Birding Trip Report Southeast Brazil: Atlantic Rainforest and Savanna, Oct-Nov 2016 The trip started in the city of Curitiba. -

South East Brazil, 18Th – 27Th January 2018, by Martin Wootton
South East Brazil 18th – 27th January 2018 Grey-winged Cotinga (AF), Pico da Caledonia – rare, range-restricted, difficult to see, Bird of the Trip Introduction This report covers a short trip to South East Brazil staying at Itororó Eco-lodge managed & owned by Rainer Dungs. Andy Foster of Serra Dos Tucanos guided the small group. Itinerary Thursday 18th January • Nightmare of a travel day with the flight leaving Manchester 30 mins late and then only able to land in Amsterdam at the second attempt due to high winds. Quick sprint (stagger!) across Schiphol airport to get onto the Rio flight which then parked on the tarmac for 2 hours due to the winds. Another roller-coaster ride across a turbulent North Atlantic and we finally arrived in Rio De Janeiro two hours late. Eventually managed to get the free shuttle to the Linx Hotel adjacent to airport Friday 19th January • Collected from the Linx by our very punctual driver (this was to be a theme) and 2.5hour transfer to Itororo Lodge through surprisingly light traffic. Birded the White Trail in the afternoon. Saturday 20th January • All day in Duas Barras & Sumidouro area. Luggage arrived. Sunday 21st January • All day at REGUA (Reserva Ecologica de Guapiacu) – wetlands and surrounding lowland forest. Andy was ill so guided by the very capable REGUA guide Adelei. Short visit late pm to Waldanoor Trail for Frilled Coquette & then return to lodge Monday 22nd January • All day around lodge – Blue Trail (am) & White Trail (pm) Tuesday 23rd January • Early start (& finish) at Pico da Caledonia. -
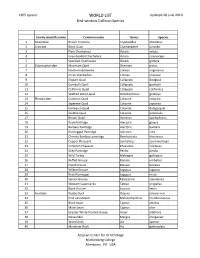
WORLD LIST Updated 28 June 2019 Bird-Window Collision Species
1305 species WORLD LIST Updated 28 June 2019 Bird-window Collision Species Family scientific name Common name Genus Species 1 Tinamidae Brown Tinamou Crypturellus obsoletus 2 Cracidae Black Guan Chamaepetes unicolor 3 Plain Chachalaca Ortalis vetula 4 Grey-headed Chachalaca Ortalis cinereiceps 5 Speckled Chachalaca Ortalis guttata 6 Odontophoridae Mountain Quail Oreortyx pictus 7 Northern Bobwhite Colinus virginianus 8 Crested Bobwhite Colinus cristatus 9 Elegant Quail Callipepla douglasii 10 Gambel's Quail Callipepla gambelii 11 California Quail Callipepla californica 12 Spotted Wood-quail Odontophorus guttatus 13 Phasianidae Common Quail Coturnix coturnix 14 Japanese Quail Coturnix japonica 15 Harlequin Quail Coturnix delegorguei 16 Stubble Quail Coturnix pectoralis 17 Brown Quail Synoicus ypsilophorus 18 Rock Partridge Alectoris graeca 19 Barbary Partridge Alectoris barbara 20 Red-legged Partridge Alectoris rufa 21 Chinese Bamboo-partridge Bambusicola thoracicus 22 Copper Pheasant Syrmaticus soemmerringii 23 Common Pheasant Phasianus colchicus 24 Grey Partridge Perdix perdix 25 Wild Turkey Meleagris gallopavo 26 Ruffed Grouse Bonasa umbellus 27 Hazel Grouse Bonasa bonasia 28 Willow Grouse Lagopus lagopus 29 Rock Ptarmigan Lagopus muta 30 Spruce Grouse Falcipennis canadensis 31 Western Capercaillie Tetrao urogallus 32 Black Grouse Lyrurus tetrix 33 Anatidae Ruddy Duck Oxyura jamaicensis 34 Pink-eared Duck Malacorhynchus membranaceus 35 Black Swan Cygnus atratus 36 Mute Swan Cygnus olor 37 Greater White-fronted Goose Anser albifrons 38 -

Adobe PDF, Job 6
Noms français des oiseaux du Monde par la Commission internationale des noms français des oiseaux (CINFO) composée de Pierre DEVILLERS, Henri OUELLET, Édouard BENITO-ESPINAL, Roseline BEUDELS, Roger CRUON, Normand DAVID, Christian ÉRARD, Michel GOSSELIN, Gilles SEUTIN Éd. MultiMondes Inc., Sainte-Foy, Québec & Éd. Chabaud, Bayonne, France, 1993, 1re éd. ISBN 2-87749035-1 & avec le concours de Stéphane POPINET pour les noms anglais, d'après Distribution and Taxonomy of Birds of the World par C. G. SIBLEY & B. L. MONROE Yale University Press, New Haven and London, 1990 ISBN 2-87749035-1 Source : http://perso.club-internet.fr/alfosse/cinfo.htm Nouvelle adresse : http://listoiseauxmonde.multimania. -

TOUR REPORT Pantanal and Interior Brazil 2018
The astonishing male Blue Finch from the rocky savannas of Brazilian Cerrado (Eduardo Patrial) PANTANAL AND INTERIOR BRAZIL 02 – 14/22 OCTOBER 2018 LEADER: EDUARDO PATRIAL This tour is always a classic and one of nicest tours in the huge Brazil. Three major biomes (Cerrado, Pantanal and Amazon) certainly guarantee lots of good birds and some spectacular mammals, besides the fantastic and scenic places, great food and friendly people, all part of this trip. And this year the Pantanal and Interior Brazil tour with a massive list of 621 species recorded, plus 27 mammals. So many good moments in field easily bring back memories from the spectacular hills from Minas Gerais and their endemics, rare and peculiar fauna; the mighty Pantanal and its abundant life, and the Mother of all Tropical forests, the great Amazon. From all wonders on this tour, best remembrances surely go to the pair of the very rare Brazilian 1 BirdQuest Tour Report: Pantanal and Interior Brazil 2018 www.birdquest-tours.com Merganser seen in the last minute (even not seen by everyone); Grey, Undulated and Tataupa Tinamous, Chestnut-bellied Guan, Red-throated and Blue-throated Piping Guans, Bare-faced and Razor-billed Curassows, Jabiru, Agami and Zigzag Herons, Black-collared, White-browed and Tiny Hawks, Mississippi Kite, Red-legged Seriema, Sunbittern, Sungrebe, Long-tailed Ground Dove, Pavonine Cuckoo, Tawny- bellied Screech Owl, Black-banded, Crested and Great Horned Owls, Great Potoo, Spot-tailed and Blackish Nightjars, Cinnamon-throated and Tapajos Hermits, the cracking -

Listado De Aves / Bird Check List Selva Misionera – Argentina – Septiembre 2019
Listado de Aves / Bird Check List Selva Misionera – Argentina – Septiembre 2019 Turismo sustentable dedicado a la conservación del Bosque Atlántico Interior. Sustainable tourism dedicated to the conservation of the Interior Atlantic Rainforest. Recopiladores- Jorge Escobar y Sergio Moya Algunas consideraciones respecto al Check -List de Aves del Refugio Privado de vida silvestre Yacutinga (2019). Nos es sumamente gratificante el hecho de poder compartir el presente listado con todos los amantes de las aves y en particular para todos los observadores que llegan a nuestra zona para deleitarse con la belleza y profusión de las Aves del norte de la Provincia de Misiones. Queremos aprovechar su interés en este listado para informarles sobre algunas características que lo componen y que consideramos de mucha importancia. 1)- Este listado sigue la nueva sistemática de la última revisión ornitológica vigente desde el año 2019, la cual considera la secuencia genética como válida. 2)- Este listado incluye una columna denominada HISTORICA. En ella están indicadas con una (H), las especies que fueron observadas en el Refugio Privado de vida silvestre Yacutinga hasta el año 2014. También se ha incluido en esta columna con una (C) aquellas especies que nos resultan registros curiosos. Nos ha parecido oportuno incluirlas en la actualidad con éstas distinciones debido a los siguientes motivos; a)- La disminución de campañas de observación destinadas específicamente a actualizar el presente listado debido a razones de índole operativa. b)- La alarmante perdida de superficie de Selva ocurrida en los alrededores de nuestra Reserva Ambiental, la cual produce indeseables impactos ambientales negativos, que seguramente han generado una dramática disminución de las especies citadas como HISTORICAS. -

Diet of Ornate Hawk-Eagle (Spizaetus Ornatus)
Revista Brasileira de Ornitologia 27(1): 31–39. ARTICLE March 2019 Diet of Ornate Hawk-Eagle (Spizaetus ornatus) Fagner Daniel Teixeira1,5, Elisa Paraíso Mesquita2, Michele Alves Ferreira3 & Felipe de Carvalho Araújo4 1 Avenida João Gonçalves Teixeira, 22, Bairro Glória, Carmópolis de Minas, MG, Brazil. 2 Rua Coronel Pedro Jorge, 26, Bairro Prado, Belo Horizonte, MG, Brazil. 3 Rua Gustavo da Silveira, 1000, Bairro Horto Florestal, Belo Horizonte, MG, Brazil. 4 Departamento de Ciências Florestais, Universidade Federal de Lavras, Lavras, MG, Brazil. 5 Corresponding author: [email protected] Received on 12 November 2018. Accepted on 21 February 2019. ABSTRACT: The Ornate Hawk-Eagle (Spizaetus ornatus) is a top predator and inhabits mainly preserved forests. It occurs from Mexico to Argentina and throughout Brazil, where it is threatened by extinction. It hunts birds, mammals and reptiles, picking up both on the ground and on the branches in the forest. Here we report data on a pair and one young individual of this species registered in the southeast of Minas Gerais state, eastern portion of the Espinhaço Range, Brazil. In addition, a literature review on the diet of the species was carried out aiming gather data on food habits. The nesting territory, as well as the nest was discovered in semi-deciduous seasonal forest area. We recorded predation of a Lesser Yellow-headed Vulture (Cathartes burrovianus) by the young. After two days of observation, the nest was overthrown, what allowed its screening for other food items discovered after analysis of some feathers and bones. Detailed records of predation of S. ornatus were non-existent or inaccurate. -

Landscape Processes Underpinning Bird Persistence and Avian-Mediated Pest Control in Fragmented Landscapes
Andrea Larissa Boesing Landscape processes underpinning bird persistence and avian-mediated pest control in fragmented landscapes São Paulo 2016 Andrea Larissa Boesing Landscape processes underpinning bird persistence and avian-mediated pest control in fragmented landscapes Persistência de aves e controle de pragas em paisagens fragmentadas – uma perspectiva da ecologia de paisagens Tese apresentada ao Instituto de Biociências da Universidade de São Paulo, para a obtenção de Título de Doutor em Ciências, na Área de Ecologia. Orientador: Jean Paul Metzger Co-orientadora: Elizabeth Nichols São Paulo 2016 FichaCatalográfica Boesing, Andrea Larissa Persistência de aves e controle de pragas em paisagens fragmentadas – uma perspectiva da ecologia de paisagens 181 páginas Tese (Doutorado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Ecologia. 1.Estrutura da paisagem 2.Cobertura florestal 3.Mata Atlântica Universidade de São Paulo. Instituto de Biociências. Departamento de Ecologia. Comissão Julgadora: Prof(a). Dr(a). Prof(a). Dr(a). Prof(a). Dr(a). Prof(a). Dr(a). Prof. Dr. Jean Paul Metzger Orientador(a) Dedication Dedico... “Àquela que sempre motivou a busca dos sonhos mais ousados, Que me ensinou a soltar as amarras, Que me mostrou como ser forte e nunca desistir. A você, meu exemplo de mulher e sabedoria, Que acompanhou o começo da jornada, mas infelizmente não o fim...” Cila Friedrich Boesing (In memorian) Epigraph Hold fast to dreams For if dreams die Life is a broken-winged bird That cannot fly. Hold fast to dreams For when dreams go Life is a barren field Frozen with snow. Langston Hughes Acknowledgments Este trabalho é resultado de um esforço conjunto que envolveu muitas pessoas.