Landscape Processes Underpinning Bird Persistence and Avian-Mediated Pest Control in Fragmented Landscapes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Birds of Reserva Ecológica Guapiaçu (REGUA)
Cotinga 33 The birds of Reserva Ecológica Guapiaçu (REGUA), Rio de Janeiro, Brazil Leonardo Pimentel and Fábio Olmos Received 30 September 2009; final revision accepted 15 December 2010 Cotinga 33 (2011): OL 8–24 published online 16 March 2011 É apresentada uma lista da avifauna da Reserva Ecológica de Guapiaçu (REGUA), uma reserva privada de 6.500 ha localizada no município de Cachoeiras de Macacu, vizinha ao Parque Estadual dos Três Picos, Estação Ecológica do Paraíso e Parque Nacional da Serra dos Órgãos, parte de um dos maiores conjuntos protegidos do Estado do Rio de Janeiro. Foram registradas um total de 450 espécies de aves, das quais 63 consideradas de interesse para conservação, como Leucopternis lacernulatus, Harpyhaliaetus coronatus, Triclaria malachitacea, Myrmotherula minor, Dacnis nigripes, Sporophila frontalis e S. falcirostris. A reserva também está desenvolvendo um projeto de reintrodução dos localmente extintos Crax blumembachii e Aburria jacutinga, e de reforço das populações locais de Tinamus solitarius. The Atlantic Forest of eastern Brazil and Some information has been published on neighbouring Argentina and Paraguay is among the birds of lower (90–500 m) elevations in the the most imperilled biomes in the world. At region10,13, but few areas have been subject to least 188 bird species are endemic to it, and 70 long-term surveys. Here we present the cumulative globally threatened birds occur there, most of them list of a privately protected area, Reserva Ecológica endemics4,8. The Atlantic Forest is not homogeneous Guapiaçu (REGUA), which includes both low-lying and both latitudinal and longitudinal gradients parts of the Serra dos Órgãos massif and nearby account for diverse associations of discrete habitats higher ground, now mostly incorporated within and associated bird communities. -

New Insights Into the Microbiota of Moth Pests
International Journal of Molecular Sciences Review New Insights into the Microbiota of Moth Pests Valeria Mereghetti, Bessem Chouaia and Matteo Montagna * ID Dipartimento di Scienze Agrarie e Ambientali, Università degli Studi di Milano, 20122 Milan, Italy; [email protected] (V.M.); [email protected] (B.C.) * Correspondence: [email protected]; Tel.: +39-02-5031-6782 Received: 31 August 2017; Accepted: 14 November 2017; Published: 18 November 2017 Abstract: In recent years, next generation sequencing (NGS) technologies have helped to improve our understanding of the bacterial communities associated with insects, shedding light on their wide taxonomic and functional diversity. To date, little is known about the microbiota of lepidopterans, which includes some of the most damaging agricultural and forest pests worldwide. Studying their microbiota could help us better understand their ecology and offer insights into developing new pest control strategies. In this paper, we review the literature pertaining to the microbiota of lepidopterans with a focus on pests, and highlight potential recurrent patterns regarding microbiota structure and composition. Keywords: symbiosis; bacterial communities; crop pests; forest pests; Lepidoptera; next generation sequencing (NGS) technologies; diet; developmental stages 1. Introduction Insects represent the most successful taxa of eukaryotic life, being able to colonize almost all environments, including Antarctica, which is populated by some species of chironomids (e.g., Belgica antarctica, Eretmoptera murphyi, and Parochlus steinenii)[1,2]. Many insects are beneficial to plants, playing important roles in seed dispersal, pollination, and plant defense (by feeding upon herbivores, for example) [3]. On the other hand, there are also damaging insects that feed on crops, forest and ornamental plants, or stored products, and, for these reasons, are they considered pests. -

The Birds of Serra Da Canastra National Park and Adjacent Areas, Minas Gerais, Brazil
The birds of Serra da Canastra National Park and adjacent areas, Minas Gerais, Brazil Luís Fábio Silveira É apresentada uma listagem da avifauna do Parque Nacional da Serra da Canastra e regiões próximas, e complementada com observações realizadas por outros autores. São relatadas algumas observações sobre espécies ameaçadas ou pouco conhecidas, bem como a extensão de distribuição para outras. Introduction Located in the south-west part of Minas Gerais state, south-east Brazil, Serra da Canastra National Park (SCNP, 71,525 ha8, 20o15'S 46o37'W)9 is regularly visited by birders as it is a well-known area in which to see cerrado specialities and a site for Brazilian Merganser Mergus octosetaceus. However, Forrester’s6 checklist constitutes the only major compilation of records from the area. Here, I list the species recorded at Serra da Canastra National Park and surrounding areas (Appendix 1), with details of threatened birds and range extensions for some species. Material and methods The dominant vegetation of Serra da Canastra National Park is cerrado sensu lato, but more than 90% of its total area is covered by subdivisions of this formation, known as campo limpo and campo sujo; the park has small portions of campo rupestre, and cerrado sensu stricto, and patches of gallery forest with Atlantic forest characteristics8. The relief is undulating, with streams in the valleys. The climate has a marked seasonality, with a rainy season in September–February and a dry season in March–August8. I studied the avifauna during February 1996–February 1998, with most fieldwork conducted in April–December 1996, when I undertook population surveys and investigated the habitat requirements of Brazilian Merganser17. -

Abstract Book
Welcome to the Ornithological Congress of the Americas! Puerto Iguazú, Misiones, Argentina, from 8–11 August, 2017 Puerto Iguazú is located in the heart of the interior Atlantic Forest and is the portal to the Iguazú Falls, one of the world’s Seven Natural Wonders and a UNESCO World Heritage Site. The area surrounding Puerto Iguazú, the province of Misiones and neighboring regions of Paraguay and Brazil offers many scenic attractions and natural areas such as Iguazú National Park, and provides unique opportunities for birdwatching. Over 500 species have been recorded, including many Atlantic Forest endemics like the Blue Manakin (Chiroxiphia caudata), the emblem of our congress. This is the first meeting collaboratively organized by the Association of Field Ornithologists, Sociedade Brasileira de Ornitologia and Aves Argentinas, and promises to be an outstanding professional experience for both students and researchers. The congress will feature workshops, symposia, over 400 scientific presentations, 7 internationally renowned plenary speakers, and a celebration of 100 years of Aves Argentinas! Enjoy the book of abstracts! ORGANIZING COMMITTEE CHAIR: Valentina Ferretti, Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA- CONICET) and Association of Field Ornithologists (AFO) Andrés Bosso, Administración de Parques Nacionales (Ministerio de Ambiente y Desarrollo Sustentable) Reed Bowman, Archbold Biological Station and Association of Field Ornithologists (AFO) Gustavo Sebastián Cabanne, División Ornitología, Museo Argentino -

Birding the Atlantic Rainforest, South-East Brazil Itororo Lodge and Regua 11Th – 20Th March 2018
BIRDING THE ATLANTIC RAINFOREST, SOUTH-EAST BRAZIL ITORORO LODGE AND REGUA 11TH – 20TH MARCH 2018 White-barred Piculet (©Andy Foster) Guided and report compiled by Andy Foster www.serradostucanos.com.br Sunday 11th March The following 10 day tour was a private trip for a group of 4 friends. We all flew in from the UK on a BA flight landing the night of the 10th and stayed in the Linx Hotel located close to the International airport in Rio de Janeiro. We met up for breakfast at 07.00 and by 08.00 our driver had arrived to take us for the 2.5 hour drive to Itororo Lodge where we were to spend our first 6 nights birding the higher elevations of the Serra do Mar Mountains. On the journey up we saw Magnificent Frigatebird, Cocoi Heron, Great White Egret, Black-crowned Night Heron, Neotropic Cormorant and Roadside Hawk. By 10.30 we had arrived at the lodge and were greeted by Bettina and Rainer who would be our hosts for the next week. The feeders were busy at the lodge and we were soon picking up new species including Azure-shouldered Tanager, Brassy-breasted Tanager, Black-goggled Tanager, Sayaca Tanager, Ruby- crowned Tanager, Golden-chevroned Tanager, Magpie Tanager, Burnished-buff Tanager, Plain Parakeet, Maroon-bellied Parakeet, Rufous-bellied Thrush, Green-winged Saltator, Pale-breasted Thrush, Violet- capped Woodnymph, Black Jacobin, Scale-throated Hermit, Sombre Hummingbird, Brazilian Ruby and White-throated Hummingbird…. not bad for the first 30 minutes! We spent the last hour or so before lunch getting to grips with the feeder birds, we also picked up brief but good views of a Black-Hawk Eagle as it flew through the lodge gardens. -

ON (19) 599-606.Pdf
SHORT COMMUNICATIONS ORNITOLOGIA NEOTROPICAL 19: 599–606, 2008 © The Neotropical Ornithological Society BODY MASSES OF BIRDS FROM ATLANTIC FOREST REGION, SOUTHEASTERN BRAZIL Iubatã Paula de Faria1 & William Sousa de Paula PPG-Ecologia, Universidade de Brasília, Brasília, DF, Brazil. E-mail: [email protected] Massa corpórea de aves da região de Floresta Atlântica, sudeste do Brasil. Key words: Neotropical birds, weight, body mass, tropical rainforest, Brazil. The Brazilian Atlantic forest is one of the In this paper, we present the values of biodiversity hotspots in the world (Myers body masses of birds captured or collected 1988, Myers et al. 2000) with high endemism using mist-nets in 17 localities in the Atlantic of bird species (Cracraft 1985, Myers et al. forest of southeastern Brazil (Table 1), 2000). Nevertheless, there is little information between 2004 and 2007. Data for sites 1, 2, 3, on the avian body masses (weight) for this and 5 were collected in January, April, August, region (Oniki 1981, Dunning 1992, Belton and December 2004; in March and April 2006 1994, Reinert et al. 1996, Sick 1997, Oniki & for sites 4, 6, 7, and 8. Others sites were col- Willis 2001, Bugoni et al. 2002) and for other lected in September 2007. The Atlantic forest Neotropical ecosystems (Fry 1970, Oniki region is composed by two major vegetation 1980, 1990; Dick et al. 1984, Salvador 1988, types: Atlantic rain forest (low to medium ele- 1990; Peris 1990, Dunning 1992, Cavalcanti & vations, = 1000 m); and Atlantic semi-decidu- Marini 1993, Marini et al. 1997, Verea et al. ous forest (usually > 600 m) (Morellato & 1999, Oniki & Willis 1999). -
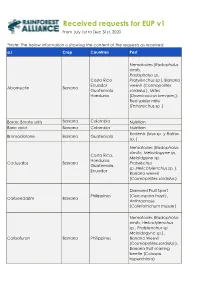
IIS 2020 Requests Overview
Received requests for EUP v1 From July 1st to Dec 31st, 2020 *Note: The below information is showing the content of the requests as received. a.i Crop Countries Pest Nematodes ( Radophulus similis, Radopholus sp, Costa Rica Pratylenchus sp ), Banana Ecuador weevil ( Cosmopolites Abamectin Banana Guatemala sordidus ), Mites Honduras (Dysmicoccus brevipes ), Red spider mite (Tetranychus sp .) Borax; Borate salts Banana Colombia Nutrition Boric acid Banana Colombia Nutrition Rodents ( Mus sp. y Rattus Bromadiolone Banana Guatemala sp. ) Nematodes ( Radopholus silmillis, Meloidogyne sp, Costa Rica, Meloidgune sp, Honduras, Cadusafos Banana Pratylechus Guatemala, sp.,Helicotylenchus sp. ), Ecuador Banana weevil (Cosmopolites sordidus ) Diamond Fruit Sport Philippines (Cercospora hayii ), Carbendazim Banana Anthracnose (Colletotrichum musae ) Nematod es (Radopholus similis, Helicotylenchus sp., Pratylenchus sp. Meloidogyne sp.) , Carbofuran Banana Philippines Banana Weevil (Cosmopolites sordidus ), Banana fruit scarring beetle (Colaspis hyperchlora) a.i Crop Countries Pest Black Sigatoka Ecuador, Costa (Mycosphaerella fijiensis ), Rica, Philippines, Yellow Sigatoka Chlorothalonil Banana Honduras, (Mycosphaerella Guatemala, musicola ), Banana Colombia Freckle ( Phyllosticta musarum ) Mealybug ( Dysmicoccus brevipes, Pseudococcus elisae, Pseudococcus sp, Ferrista sp ), Dysmicoccus sp , Scale insects (Aspidiotus destructor, Diaspis boisduvalii ), Thrips (Thrips florum, Franckliniella spp, Chaetanaphothrips signipennis ), Banana fruit Philippines, -

Guia Para Observação Das Aves Do Parque Nacional De Brasília
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/234145690 Guia para observação das aves do Parque Nacional de Brasília Book · January 2011 CITATIONS READS 0 629 4 authors, including: Mieko Kanegae Fernando Lima Favaro Federal University of Rio de Janeiro Instituto Chico Mendes de Conservação da Bi… 7 PUBLICATIONS 74 CITATIONS 17 PUBLICATIONS 69 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Fernando Lima Favaro on 28 May 2014. The user has requested enhancement of the downloaded file. Brasília - 2011 GUIA PARA OBSERVAÇÃO DAS AVES DO PARQUE NACIONAL DE BRASÍLIA Aílton C. de Oliveira Mieko Ferreira Kanegae Marina Faria do Amaral Fernando de Lima Favaro Fotografia de Aves Marcelo Pontes Monteiro Nélio dos Santos Paulo André Lima Borges Brasília, 2011 GUIA PARA OBSERVAÇÃO DAS AVES DO APRESENTAÇÃO PARQUE NACIONAL DE BRASÍLIA É com grande satisfação que apresento o Guia para Observação REPÚblica FEDERATiva DO BRASIL das Aves do Parque Nacional de Brasília, o qual representa um importante instrumento auxiliar para os observadores de aves que frequentam ou que Presidente frequentarão o Parque, para fins de lazer (birdwatching), pesquisas científicas, Dilma Roussef treinamentos ou em atividades de educação ambiental. Este é mais um resultado do trabalho do Centro Nacional de Pesquisa e Vice-Presidente Conservação de Aves Silvestres - CEMAVE, unidade descentralizada do Instituto Michel Temer Chico Mendes de Conservação da Biodiversidade (ICMBio) e vinculada à Diretoria de Conservação da Biodiversidade. O Centro tem como missão Ministério do Meio Ambiente - MMA subsidiar a conservação das aves brasileiras e dos ambientes dos quais elas Izabella Mônica Vieira Teixeira dependem. -

Cavity Characteristics, but Not Habitat, Influence Nest Survival Of
Biological Conservation 184 (2015) 193–200 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Cavity characteristics, but not habitat, influence nest survival of cavity-nesting birds along a gradient of human impact in the subtropical Atlantic Forest ⇑ Kristina L. Cockle a,b,c, , Alejandro Bodrati b, Martjan Lammertink b,d,e, Kathy Martin c,f a School of Renewable Natural Resources, Louisiana State University and LSU AgCenter, Baton Rouge, LA 70803, USA b Proyecto Selva de Pino Paraná, Vélez Sarsfield y San Jurjo S/N (3352), San Pedro, Misiones, Argentina c Centre for Applied Conservation Research, Department of Forest and Conservation Sciences, University of British Columbia, 2424 Main Mall, Vancouver, BC V6T 1Z4, Canada d CICyTTP-CONICET, Materi y España S/N (3105), Diamante, Entre Ríos, Argentina e Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14850, USA f Environment Canada, 5421 Robertson Road, RR1, Delta, BC V4K 3N2, Canada article info abstract Article history: Cavity-nesting vertebrates are an important component of biodiversity in tropical and subtropical forests, Received 6 November 2014 but their persistence will increasingly depend on remnant trees in logged forest and agricultural areas. To Received in revised form 20 January 2015 identify key habitat features for conservation, we examined the factors that influenced daily nest survival Accepted 26 January 2015 for a community of cavity-nesting birds along a gradient of human impact, from primary Atlantic Forest through logged forest to farms. We used logistic-exposure models to determine how characteristics of the habitat, nest tree, cavity, and timing influenced daily nest survival. -

Rochely Santos Morandini
Rochely Santos Morandini Diversidade funcional das aves do Cerrado com simulações da perda de fisionomias campestres e de espécies ameaçadas: implicações para a conservação. (VERSÃO CORRIGIDA – versão original disponível na Biblioteca do IB-USP e na Biblioteca Digital de Teses e Dissertações (BDTD) da USP) Functional Diversity of Cerrado birds with a simulation of the loss of open areas and endangered species: implications for conservation. São Paulo 2013 Rochely Santos Morandini Diversidade funcional das aves do Cerrado com simulações da perda de fisionomias campestres e de espécies ameaçadas: implicações para a conservação. Functional Diversity of Cerrado birds with a simulation of the loss of open areas and endangered species: implications for conservation. Dissertação apresentada ao Instituto de Biociências da Universidade de São Paulo para a obtenção do Título de Mestre em Ciências, na Área de Ecologia. Orientador: Prof. Dr. José Carlos Motta Junior. São Paulo 2013 Morandini, Rochely Santos Diversidade funcional das aves do Cerrado com simulações da perda de fisionomias campestres e de espécies ameaçadas: implicações para conservação. 112 páginas Dissertação (Mestrado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Ecologia. 1. Aves 2. Cerrado 3. Diversidade Funcional I. Universidade de São Paulo. Instituto de Biociências. Departamento de Ecologia Comitê de Acompanhamento: Luís Fábio Silveira Marco Antônio P. L. Batalha Comissão Julgadora: ________________________ ________________________ Prof(a). Dr. Marco Ant ônio Prof(a). Dr. Sergio Tadeu Meirelles Monteiro Granzinolli ____________________________________ Orientador: Prof. Dr. José Carlos Motta Junior Dedicatória A melhor lembrança que tenho da infância são as paisagens de minha terra natal. Dedico este estudo ao Cerrado, com seus troncos retorcidos, seu amanhecer avermelhado, paisagens onde habitam aves tão encantadoras que me tonteiam. -

Southeastern Brazil: Best of the Atlantic Forest
SOUTHEASTERN BRAZIL: BEST OF THE ATLANTIC FOREST OCTOBER 21–NOVEMBER 5, 2018 Green-crowned Plovercrest (©Kevin J. Zimmer) LEADERS: KEVIN ZIMMER & RICARDO BARBOSA LIST COMPILED BY: KEVIN ZIMMER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM SOUTHEASTERN BRAZIL: BEST OF THE ATLANTIC FOREST October 21–November 5, 2018 By Kevin Zimmer Once again, our Southeastern Brazil tour delivered the bonanza of Atlantic Forest endemics and all-around great birding that we have come to expect from this region. But no two trips are ever exactly alike, and, as is always the case, the relative success of this tour in any given year, at least as measured in total species count and number of endemics seen, comes down to weather. And as we all know, the weather isn’t what it used to be, anywhere! We actually experienced pretty typical amounts of rain this year, and although it no doubt affected our birding success to some extent, its overall impact was relatively minimal. Nonetheless, we tallied 410 species , a whopping 150 of which were regional and/or Brazilian endemics! These figures become all the more impressive when you consider that 47 of the wider ranging species not included as “endemics” in the preceding tallies are represented in southeast Brazil by distinctive subspecies endemic to the Atlantic Forest region, and that at least 15–20 of these subspecies that we recorded during our tour are likely to be elevated to separate species status in the near future. We convened in mid-morning at the hotel in São Paulo and then launched into the five- hour drive to Intervales State Park, my own personal favorite among the many great spots in southeast Brazil. -

Northeast Brazil
Northeast Brazil 10th December 2017 – 10th January 2018 Sjoerd Radstaak [email protected] Introduction This trip report describes a four-week-trip to Northeast-Brazil, together with good friends Pieter van Veelen, Jelmer Poelstra and Lars Buckx. For three of us, it was our first time of proper birding in South-America. Only Jelmer had been in Ecuador, Peru and Colombia before, but never in this part of the continent. Sjoerd did visit NE Brazil in December 2018 again as a tour leader for BirdingBreaks.nl. Based on the high degree of endemism, combined with the fact many of these endemics are critically endangered, we decided to visit NE-Brazil. Also, it appears to be a good destination to become familiar with the bird families of South-America without getting overwhelmed by the number of species. While Brazil has always been a birder’s destination, the north-eastern part of the country has long been the forgotten corner of Brazil. However, more than 15 birds new to science have been described here over the last 20 years. Of those, the most famous is - of course - Araripe Manakin: only discovered in 1996 and first described in 1998. Even today avian (re)discoveries are ‘regular’ here, for example the discovery of Blue-eyed ground-Dove in 2016 – a species that was believed to be extinct, as it had last been seen in 1941! Others include Alagoas Foliage-Gleaner, Orange-bellied Antwren and Pinto’s Spinetail. Sadly enough, many of these newly described species and others are now critically endangered or even extinct – with Alagoas Foliage-Gleaner last seen in 2012.