New Insights Into the Microbiota of Moth Pests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pests and Diseases
Clivia assassins – pests and diseases Clivia is really quite a resistant little genus, with only a few serious pests and diseases that can be life threatening . Others, though not lethal, can seriously affect a plant’s appearance and growth . General hygienic culture conditions like good drainage, removal of infected plants and material and a spray programme can successfully prevent these pests and diseases from becoming a serious problem . Just a note of caution when working with toxic chemicals: Read the instructions of all chemicals before use! Make sure that chemicals can be mixed without detrimental effects to treated plants – if not stated as mixable, test it first on a few plants before you treat your whole collection . Plant pests Lily borer (Brithys crini, Brithys pancratii) Brithys species, also known as Amaryllis caterpillars, are serious pests amongst members of the Amaryllidaceae. They target Crinum, Cyrthanthus, Haemanthus, Nerine, Amaryllis and Clivia, to name a few. This destructive pest has three to four generations in nature annually and if a severe infestation occurs it can destroy plants within a few days. The caterpillar Breakfast! The yellow and brown-black banding is easily distinguishable with its yellow and black or brown pattern of the Brithys caterpillar makes it easy to recognise . Only a mushy pseudo stem remains banding pattern. Young larvae emerge from a cluster of after Mr Caterpillar’s visit . Growing clivias eggs, usually on the underside (abaxial side) of leaves, and then start to tunnel into the leaf. Once inside, the larvae eat the soft tissue between the outer two epidermal cell layers, tunnelling their way towards the base of the leaf. -

Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus Sp
fmicb-07-01005 June 24, 2016 Time: 16:20 # 1 ORIGINAL RESEARCH published: 28 June 2016 doi: 10.3389/fmicb.2016.01005 The Generalist Inside the Specialist: Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus sp. Cristina Vilanova1,2, Joaquín Baixeras1, Amparo Latorre1,2,3* and Manuel Porcar1,2* 1 Cavanilles Institute of Biodiversity and Evolutionary Biology, Universitat de València, Valencia, Spain, 2 Institute for Integrative Systems Biology (I2SysBio), University of Valencia-CSIC, Valencia, Spain, 3 Unidad Mixta de Investigación en Genómica y Salud, Centro Superior de Investigación en Salud Pública, Valencia, Spain Some specialist insects feed on plants rich in secondary compounds, which pose a major selective pressure on both the phytophagous and the gut microbiota. However, microbial communities of toxic plant feeders are still poorly characterized. Here, we Edited by: Mark Alexander Lever, show the bacterial communities of the gut of two specialized Lepidoptera, Hyles ETH Zürich, Switzerland euphorbiae and Brithys crini, which exclusively feed on latex-rich Euphorbia sp. and Reviewed by: alkaloid-rich Pancratium maritimum, respectively. A metagenomic analysis based on Virginia Helena Albarracín, CONICET, Argentina high-throughput sequencing of the 16S rRNA gene revealed that the gut microbiota Jeremy Dodsworth, of both insects is dominated by the phylum Firmicutes, and especially by the common California State University, gut inhabitant Enterococcus sp. Staphylococcus sp. are also found in H. euphorbiae San Bernardino, USA though to a lesser extent. By scanning electron microscopy, we found a dense ring- *Correspondence: Manuel Porcar shaped bacterial biofilm in the hindgut of H. euphorbiae, and identified the most [email protected]; prominent bacterium in the biofilm as Enterococcus casseliflavus through molecular Amparo Latorre [email protected] techniques. -

Meta-Omics Tools in the World of Insect-Microorganism Interactions
biology Review Meta-Omics Tools in the World of Insect-Microorganism Interactions Antonino Malacrinò Department of Physics, Chemistry and Biology (IFM), Linköping University, 58183 Linköping, Sweden; [email protected] or [email protected] Received: 25 October 2018; Accepted: 22 November 2018; Published: 27 November 2018 Abstract: Microorganisms are able to influence several aspects of insects’ life, and this statement is gaining increasing strength, as research demonstrates it daily. At the same time, new sequencing technologies are now available at a lower cost per base, and bioinformatic procedures are becoming more user-friendly. This is triggering a huge effort in studying the microbial diversity associated to insects, and especially to economically important insect pests. The importance of the microbiome has been widely acknowledged for a wide range of animals, and also for insects this topic is gaining considerable importance. In addition to bacterial-associates, the insect-associated fungal communities are also gaining attention, especially those including plant pathogens. The use of meta-omics tools is not restricted to the description of the microbial world, but it can be also used in bio-surveillance, food safety assessment, or even to bring novelties to the industry. This mini-review aims to give a wide overview of how meta-omics tools are fostering advances in research on insect-microorganism interactions. Keywords: metagenomics; metatranscriptomics; metaproteomics; microbiome; insect pests 1. Introduction It is widely acknowledged that microorganisms are the main drivers of several fundamental physical, chemical and biological phenomena [1]. As scientific techniques and instruments evolve, the role of microorganisms in shaping the lifestyle of other organisms becomes clearer. -

Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier Area, Swellendam
Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier area, Swellendam by Johannes Philippus Groenewald Thesis presented in fulfilment of the requirements for the degree of Masters in Science in Conservation Ecology in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Michael J. Samways Co-supervisor: Dr. Ruan Veldtman December 2014 Stellenbosch University http://scholar.sun.ac.za Declaration I hereby declare that the work contained in this thesis, for the degree of Master of Science in Conservation Ecology, is my own work that have not been previously published in full or in part at any other University. All work that are not my own, are acknowledge in the thesis. ___________________ Date: ____________ Groenewald J.P. Copyright © 2014 Stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Acknowledgements Firstly I want to thank my supervisor Prof. M. J. Samways for his guidance and patience through the years and my co-supervisor Dr. R. Veldtman for his help the past few years. This project would not have been possible without the help of Prof. H. Geertsema, who helped me with the identification of the Lepidoptera and other insect caught in the study area. Also want to thank Dr. K. Oberlander for the help with the identification of the Oxalis species found in the study area and Flora Cameron from CREW with the identification of some of the special plants growing in the area. I further express my gratitude to Dr. Odette Curtis from the Overberg Renosterveld Project, who helped with the identification of the rare species found in the study area as well as information about grazing and burning of Renosterveld. -
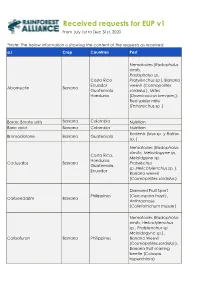
IIS 2020 Requests Overview
Received requests for EUP v1 From July 1st to Dec 31st, 2020 *Note: The below information is showing the content of the requests as received. a.i Crop Countries Pest Nematodes ( Radophulus similis, Radopholus sp, Costa Rica Pratylenchus sp ), Banana Ecuador weevil ( Cosmopolites Abamectin Banana Guatemala sordidus ), Mites Honduras (Dysmicoccus brevipes ), Red spider mite (Tetranychus sp .) Borax; Borate salts Banana Colombia Nutrition Boric acid Banana Colombia Nutrition Rodents ( Mus sp. y Rattus Bromadiolone Banana Guatemala sp. ) Nematodes ( Radopholus silmillis, Meloidogyne sp, Costa Rica, Meloidgune sp, Honduras, Cadusafos Banana Pratylechus Guatemala, sp.,Helicotylenchus sp. ), Ecuador Banana weevil (Cosmopolites sordidus ) Diamond Fruit Sport Philippines (Cercospora hayii ), Carbendazim Banana Anthracnose (Colletotrichum musae ) Nematod es (Radopholus similis, Helicotylenchus sp., Pratylenchus sp. Meloidogyne sp.) , Carbofuran Banana Philippines Banana Weevil (Cosmopolites sordidus ), Banana fruit scarring beetle (Colaspis hyperchlora) a.i Crop Countries Pest Black Sigatoka Ecuador, Costa (Mycosphaerella fijiensis ), Rica, Philippines, Yellow Sigatoka Chlorothalonil Banana Honduras, (Mycosphaerella Guatemala, musicola ), Banana Colombia Freckle ( Phyllosticta musarum ) Mealybug ( Dysmicoccus brevipes, Pseudococcus elisae, Pseudococcus sp, Ferrista sp ), Dysmicoccus sp , Scale insects (Aspidiotus destructor, Diaspis boisduvalii ), Thrips (Thrips florum, Franckliniella spp, Chaetanaphothrips signipennis ), Banana fruit Philippines, -

Biodiversiteitsopname Biodiversity Assessment
Biodiversiteitsopname Biodiversity Assessment Bome - Trees (77 sp) Veldblomme - Flowering veld plants (65 sp) Grasse - Grasses (41 sp) Naaldekokers - Dragonflies (46 sp) Skoenlappers - Butterflies (81 sp) Motte - Moths (95 sp) Nog insekte - Other insects (102 sp) Spinnekoppe - Spiders (53 sp) Paddas - Frogs (14 sp) Reptiele - Reptiles (22 sp) Voëls - Birds (185 sp) Soogdiere - Mammals (23 sp) 4de uitgawe: Jan 2015 Plante/Plants Diere/Animals (24 000 spp in SA) Anthropoda Chordata (>150 000 spp in SA) Arachnida Insecta (spinnekoppe/spiders, 2020 spp in SA) Neuroptera – mayflies, lacewings, ant-lions (385 spp in SA) Odonata – dragonflies (164 spp in SA) Blattodea – cockroaches (240 spp in SA) Mantodea – mantids (185 spp in SA) Isoptera – termites (200 spp in SA) Orthoptera – grasshoppers, stick insects (900 spp in SA) Phthiraptera – lice (1150 spp in SA) Hemiptera – bugs (>3500 spp in SA) Coleoptera – beetles (18 000 spp in SA) Lepidoptera – butterflies (794 spp in SA), moths (5200 spp in SA) Diptera – flies (4800 spp in SA) Siphonoptera – fleas (100 spp in SA) Hymenoptera – ants, bees, wasps (>6000 spp in SA) Trichoptera – caddisflies (195 spp in SA) Thysanoptera – thrips (230 spp in SA) Vertebrata Tunicata (sea creatures, etc) Fish Amphibia Reptiles Birds Mammals (115 spp in SA) (255 spp in SA) (858 spp in SA) (244 spp in SA) Bome – Trees (n=77) Koffiebauhinia - Bauhinia petersiana - Dainty bauhinia Rooi-ivoor - Berchemia zeyheri - Red ivory Witgat - Boscia albitrunca - Shepherd’s tree Bergvaalbos - Brachylaena rotundata - Mountain silver-oak -

Diversity of the Moth Fauna (Lepidoptera: Heterocera) of a Wetland Forest: a Case Study from Motovun Forest, Istria, Croatia
PERIODICUM BIOLOGORUM UDC 57:61 VOL. 117, No 3, 399–414, 2015 CODEN PDBIAD DOI: 10.18054/pb.2015.117.3.2945 ISSN 0031-5362 original research article Diversity of the moth fauna (Lepidoptera: Heterocera) of a wetland forest: A case study from Motovun forest, Istria, Croatia Abstract TONI KOREN1 KAJA VUKOTIĆ2 Background and Purpose: The Motovun forest located in the Mirna MITJA ČRNE3 river valley, central Istria, Croatia is one of the last lowland floodplain 1 Croatian Herpetological Society – Hyla, forests remaining in the Mediterranean area. Lipovac I. n. 7, 10000 Zagreb Materials and Methods: Between 2011 and 2014 lepidopterological 2 Biodiva – Conservation Biologist Society, research was carried out on 14 sampling sites in the area of Motovun forest. Kettejeva 1, 6000 Koper, Slovenia The moth fauna was surveyed using standard light traps tents. 3 Biodiva – Conservation Biologist Society, Results and Conclusions: Altogether 403 moth species were recorded Kettejeva 1, 6000 Koper, Slovenia in the area, of which 65 can be considered at least partially hygrophilous. These results list the Motovun forest as one of the best surveyed regions in Correspondence: Toni Koren Croatia in respect of the moth fauna. The current study is the first of its kind [email protected] for the area and an important contribution to the knowledge of moth fauna of the Istria region, and also for Croatia in general. Key words: floodplain forest, wetland moth species INTRODUCTION uring the past 150 years, over 300 papers concerning the moths Dand butterflies of Croatia have been published (e.g. 1, 2, 3, 4, 5, 6, 7, 8). -

The Effects of Climate Change on the Pests and Diseases of Coffee
y & W log ea to th a e im r l F o Journal of Climatology & Weather C f r e o c l a a s n Groenen, Climatol Weather Forecasting 2018, 6:3 t r i n u g o J Forecasting DOI: 10.4172/2332-2594.1000239 ISSN: 2332-2594 Review Article Open Access The Effects of Climate Change on the Pests and Diseases of Coffee Crops in Mesoamerica Danielle Groenen* Department of Earth, Ocean and Atmospheric Science and Center for Ocean–Atmospheric Prediction Studies, Florida State University, Florida, United States *Corresponding author: Danielle Groenen, Department of Earth, Ocean and Atmospheric Science and Center for Ocean–Atmospheric Prediction Studies, Florida State University, Florida, United States, Tel: 18506442525; E-mail: [email protected] Received Date: August 30, 2018; Accepted Date: September 21, 2018; Published Date: September 26, 2018 Copyright: © 2018 Groenen D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Coffee is an in-demand commodity that is being threatened by climate change. Increasing temperatures and rainfall variability are predicted in the region of Mexico and Central America (Mesoamerica). This region is plagued with pests and diseases that have already caused millions of dollars in damages and losses to the coffee industry. This paper examines three pests that negatively affect coffee plants: the coffee borer beetle, the black twig borer, and nematodes. In addition, this paper examines three diseases that can destroy coffee crops: bacterial blight, coffee berry disease, and coffee leaf rust. -

Download Article (PDF)
Biologia 66/2: 288—293, 2011 Section Cellular and Molecular Biology DOI: 10.2478/s11756-011-0021-6 The first investigation of the diversity of bacteria associated with Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Hacer Muratoglu, Zihni Demirbag &KazimSezen* Karadeniz Technical University, Faculty of Arts and Sciences, Department of Biology, 61080 Trabzon, Turkey; e-mail: [email protected] Abstract: Colorado potato beetle, Leptinotarsa decemlineata (Say), is a devastating pest of potatoes in North America and Europe. L. decemlineata has developed resistance to insecticides used for its control. In this study, in order to find a more effective potential biological control agent against L. decemlineata, we investigated its microbiota and tested their insecticidal effects. According to morphological, physiological and biochemical tests as well as 16S rDNA sequences, microbiota was identified as Leclercia adecarboxylata (Ld1), Acinetobacter sp. (Ld2), Acinetobacter sp. (Ld3), Pseudomonas putida (Ld4), Acinetobacter sp. (Ld5) and Acinetobacter haemolyticus (Ld6). The insecticidal activities of isolates at 1.8×109 bacteria/mL dose within five days were 100%, 100%, 35%, 100%, 47% and 100%, respectively, against the L. decemlineata larvae. The results indicate that Leclercia adecarboxylata (Ld1) and Pseudomonas putida (Ld4) isolates may be valuable potential biological control agents for biological control of L. decemlineata. Key words: Leptinotarsa decemlineata; 16S rDNA; microbiota; insecticidal activity; microbial control. Abbreviations: ANOVA, one-way analysis of variance; LSD, least significant difference; PBS, phosphate buffer solution. Introduction used because of marketing concerns and limited num- ber of transgenic varieties available. Also, recombinant Potato is an important crop with ∼4.3 million tons defence molecules in plants may affect parasitoids or of production on 192,000 hectares of growing area predators indirectly (Bouchard et al. -

Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use
Int. J. Mol. Sci. 2013, 14, 11713-11741; doi:10.3390/ijms140611713 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use Adam M. Takos and Fred Rook * Plant Biochemistry Laboratory, Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg, Denmark; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +45-3533-3343; Fax: +45-3533-3300. Received: 28 April 2013; in revised form: 26 May 2013 / Accepted: 27 May 2013 / Published: 31 May 2013 Abstract: The alkaloids characteristically produced by the subfamily Amaryllidoideae of the Amaryllidaceae, bulbous plant species that include well know genera such as Narcissus (daffodils) and Galanthus (snowdrops), are a source of new pharmaceutical compounds. Presently, only the Amaryllidaceae alkaloid galanthamine, an acetylcholinesterase inhibitor used to treat symptoms of Alzheimer’s disease, is produced commercially as a drug from cultivated plants. However, several Amaryllidaceae alkaloids have shown great promise as anti-cancer drugs, but their further clinical development is restricted by their limited commercial availability. Amaryllidaceae species have a long history of cultivation and breeding as ornamental bulbs, and phytochemical research has focussed on the diversity in alkaloid content and composition. In contrast to the available pharmacological and phytochemical data, ecological, physiological and molecular aspects of the Amaryllidaceae and their alkaloids are much less explored and the identity of the alkaloid biosynthetic genes is presently unknown. An improved molecular understanding of Amaryllidaceae alkaloid biosynthesis would greatly benefit the rational design of breeding programs to produce cultivars optimised for the production of pharmaceutical compounds and enable biotechnology based approaches. -

BRUNSVIGIA ORIENTALIS Candelabra flower, Koningskandelaar, Perdespookbossie Amaryllidaceae
SNR FACT SHEET BRUNSVIGIA ORIENTALIS Candelabra flower, Koningskandelaar, Perdespookbossie Amaryllidaceae Late summer in Steenbok Park sees the emergence of the spectacular crimson Candelabra flower or Brunsvigia orientalis which grows in scattered colonies in coastal sand. The bud of this large bulb pushes up through the sand on its sturdy stem before a leaf can be seen, and produces up to 40 flowers in a head shaped like a rounded candelabra. As the flowers fade the ovaries enlarge and become papery and eventually the flower stem breaks away and the flower head is blown about, tumbling over the ground and scattering its seeds. These ‘balls’ blowing in the wind no doubt give rise to the Afrikaans name Perdespookbossie. The plant was initially called Amaryllis orientalis, but in 1753 Lorenz Heister, a botanist and professor of medicine at the University of Helmstädt, renamed it Brunsvigia in honour of his patron the Duke of Brunswick. Karl Wilhelm Ferdinand (1735-1806), a cultured and benevolent despot, promoted the study of plants. The bulb had been sent to Germany in 1748 by Cape Governor, Ryk Tulbagh, who was very interested in the flora and fauna of the Cape and regularly sent plants and stuffed animals to Europe. Brunsvigias are deciduous and have adapted to the dry period of the year by resting underground. The large flower heads appear shortly before the rainy season. Sunbirds searching for nectar in the tubular flowers are their chief pollinators. Once the seeds have been scattered they germinate very quickly, giving the seedling a full rainy season to develop sufficiently to withstand its first dry season underground. -

Coffee Plant the Coffee Plant Makes a Great Indoor, Outdoor Shade, Or Office Plant
Coffee Plant The coffee plant makes a great indoor, outdoor shade, or office plant. Water when dry or the plant will let you know when it droops. Do not let it sit in water so tip over the pot if you over water the plant. Preform the finger test to check for dryness. When the plant is dry about an inch down, water thoroughly. The plant will stay pot bound about two years at which time you will transplant and enjoy a beautiful ornamental plant. See below. Coffea From Wikipedia, the free encyclopedia This article is about the biology of coffee. For the beverage, see Coffee. Coffea Coffea arabica trees in Brazil Scientific classification Kingdom: Plantae (unranked): Angiosperms (unranked): Eudicots (unranked): Asterids Order: Gentianales Family: Rubiaceae Subfamily: Ixoroideae Tribe: Coffeeae[1] Genus: Coffea L. Type species Coffea arabica L.[2] Species Coffea ambongensis Coffea anthonyi Coffea arabica - Arabica Coffee Coffea benghalensis - Bengal coffee Coffea boinensis Coffea bonnieri Coffea canephora - Robusta coffee Coffea charrieriana - Cameroonian coffee - caffeine free Coffea congensis - Congo coffee Coffea dewevrei - Excelsa coffee Coffea excelsa - Liberian coffee Coffea gallienii Coffea liberica - Liberian coffee Coffea magnistipula Coffea mogeneti Coffea stenophylla - Sierra Leonian coffee Coffea canephora green beans on a tree in Goa, India. Coffea is a large genus (containing more than 90 species)[3] of flowering plants in the madder family, Rubiaceae. They are shrubs or small trees, native to subtropical Africa and southern Asia. Seeds of several species are the source of the popular beverage coffee. After their outer hull is removed, the seeds are commonly called "beans".