Series Chemistry and Technology 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
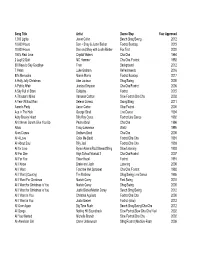
Music List by Year
Song Title Artist Dance Step Year Approved 1,000 Lights Javier Colon Beach Shag/Swing 2012 10,000 Hours Dan + Shay & Justin Bieber Foxtrot Boxstep 2019 10,000 Hours Dan and Shay with Justin Bieber Fox Trot 2020 100% Real Love Crystal Waters Cha Cha 1994 2 Legit 2 Quit MC Hammer Cha Cha /Foxtrot 1992 50 Ways to Say Goodbye Train Background 2012 7 Years Luke Graham Refreshments 2016 80's Mercedes Maren Morris Foxtrot Boxstep 2017 A Holly Jolly Christmas Alan Jackson Shag/Swing 2005 A Public Affair Jessica Simpson Cha Cha/Foxtrot 2006 A Sky Full of Stars Coldplay Foxtrot 2015 A Thousand Miles Vanessa Carlton Slow Foxtrot/Cha Cha 2002 A Year Without Rain Selena Gomez Swing/Shag 2011 Aaron’s Party Aaron Carter Slow Foxtrot 2000 Ace In The Hole George Strait Line Dance 1994 Achy Breaky Heart Billy Ray Cyrus Foxtrot/Line Dance 1992 Ain’t Never Gonna Give You Up Paula Abdul Cha Cha 1996 Alibis Tracy Lawrence Waltz 1995 Alien Clones Brothers Band Cha Cha 2008 All 4 Love Color Me Badd Foxtrot/Cha Cha 1991 All About Soul Billy Joel Foxtrot/Cha Cha 1993 All for Love Byran Adams/Rod Stewart/Sting Slow/Listening 1993 All For One High School Musical 2 Cha Cha/Foxtrot 2007 All For You Sister Hazel Foxtrot 1991 All I Know Drake and Josh Listening 2008 All I Want Toad the Wet Sprocket Cha Cha /Foxtrot 1992 All I Want (Country) Tim McGraw Shag/Swing Line Dance 1995 All I Want For Christmas Mariah Carey Fast Swing 2010 All I Want for Christmas is You Mariah Carey Shag/Swing 2005 All I Want for Christmas is You Justin Bieber/Mariah Carey Beach Shag/Swing 2012 -

1 16 Elkland Road ** DANCE MUSIC/ TOP 40 ** HEY HO – THE
16 Elkland Road Melville, NY 11747 631-643-2561 631-643-2563 Fax www.CreationsMusic.com ** DANCE MUSIC/ TOP 40 ** HEY HO – THE LUMINEERS CLARITY - ZEDD GET LUCKY – DAFT PUNK FEAT. PHARRELL I WILL WAIT – MUMFORD & SONS (RED ROCKS VERSION) LITTLE TALKS – OF MONSTERS AND MEN FEEL THIS MOMENT – PITBULL FEAT. CHRISTINA AGUILERA DIAMONDS – RIHANNA STAY - RIHANNA HOME – PHILLIP PHILLIPS DIE YOUNG – KESHA GIRL ON FIRE –ALICIA KEYS DAYLIGHT – MAROON 5 DRIVE BY- TRAIN THE A TEAM – ED SHEERAN LOCKED OUT OF HEAVEN – BRUNO MARS DON’T YOU WORRY CHILD – SWEEDISH HOUSE MAFIA SWEET NOTHING – CALVIN HARRIS/FLORENCE WELCH JUST GIVE ME A REASON – PINK FEAT. FUN CALL ME MAYBE – CARLY RAE JEPSEN GLAD YOU CAME – THE WANTED GOTYE – SOMEBODY THAT I USED TO KNOW TITANIUM – DAVID GUETTA & SIA WILD ONES – SIA/FLO-RIDA FEEL SO CLOSE – CALVIN HARRIS TURN ME ON – NIKKI MINAJ MR. SAXOBEAT – ALEXANDRA STAN GOOD LIFE – ONE REPUBLIC PITBULL/NE-YO – GIVE ME EVERYTHING SOMEONE LIKE YOU – ADELE MOVES LIKE JAGGER – MAROON 5 ONE MORE NIGHT – MAROON 5 DEV- DANCING IN THE DARK PUMPED UP KICKS – FOSTER THE PEOPLE 1 SET FIRE TO THE RAIN – ADELE ROLLING IN THE DEEP – ADELE SOMEONE LIKE YOU – ADELE TURNING TABLES - ADELE FORGET YOU – CEE LO GREEN PARTY ROCK - LMFAO CLUB CANT HANDLE ME – FLO RIDA IN THE DARK - DEV ON THE FLOOR – JLO & PITBULL GRENADE – BRUNO MARS JUST THE WAY YOU ARE – BRUNO MARS VALERI- AMY WINEHOUSE WILL YOU STILL LOVE ME – AMY WINEHOUSE O.M.G – USHER RAISE YOUR GLASS – PINK LOVE THE WAY YOU LIE – EMINEM & RIHANNA NEED YOU NOW – LADY ANTEBELLUM STEREO LOVE – EDWARD MAYA I GOT A FEELING – BLACK EYED PEAS WE FOUND LOVE –RIHANNA ONLY GIRL IN THE WORLD – RIHANNA S & M – RIHANNA DON’T STOP THE MUSIC – RIHANNA MEET ME HALFWAY – BLACK EYED PEAS EMPIRE STATE – JAY Z / ALICIA KEYS CALIFORNIA GIRLS – KATY PERY TEENAGE DREAM – KATY PERRY T.G.I.F. -

Name Artist Releaseddecade Genre Can't Stop the Feeling Justin Timberlake 2016 Disco Pop Hello Adele 2015 Soul Cake by the Ocean DNCE 2015 Funk Disco S.O.B
name artist releaseddecade genre Can't Stop The Feeling Justin Timberlake 2016 Disco Pop Hello Adele 2015 Soul Cake By The Ocean DNCE 2015 Funk Disco S.O.B. Nathaniel Rateliff and the Night Sweats 2015 Rock All You Need Is Love Rae Morris 2015 Pop Can't Feel My Face The Weeknd 2015 Pop Uptown Funk Bruno Mars 2014 Funk Thinking Out Loud Ed Sheeran 2014 Soul One Great Mystery Lady Antebellum 2014 Ballad I Don't Dance Lee Brice 2014 Country All About The Bass Meghan Trainor 2014 Pop Lips Are Movin Meghan Trainor 2014 Pop Fireball Pitbulll Feat John Ryan 2014 Samba Shake It Off Taylor Swift 2014 Pop Shut Up And Dance Walk The Moon 2014 New Wave Treasure Bruno Mars 2013 Funk Get Lucky Daft Punk 2013 Disco Come to Me Goo Goo Dolls 2013 Alternative Roar Katy Perry 2013 Pop Hey Pretty Girl Kip Moore 2013 Country Young and Beautiful Lana Del Rey 2013 Ballad Happy Pharrell Williams 2013 Funk Timber Pitbull & Kesha 2013 Dance Pop Blurred Lines Robin Thick 2013 Disco My Baby Girl Sol Knopf 2013 Contemporary Locked Out Of Heaven Bruno Mars 2012 Funk I'm Shakin Jack White 2012 Rock I Won't Give Up Jason Mraz 2012 Pop Rock Ho Hey The Lumineers 2012 Indie Folk Count on Me Bruno Mars 2011 Folk Rock Marry You Bruno Mars 2011 Pop Feel So Close Calvin Harris 2011 House Call Me Maybe Carly Rae 2011 Teen Pop A Thousand Years Christina Perri 2011 Pop Good Feeling Flo Rida 2011 Hip House Last Friday Night (T.G.I.F.) Katy Perry 2011 Electropop The Edge of Glory Lady Gaga 2011 Electropop You and I Lady Gaga 2011 Rock Party Rock Anthem LMFAO 2011 Electrohop Moves -

Top 40 ** Hey Mama – Feat
16 Elkland Road Melville, NY 11747 631-643-2561 631-643-2563 Fax www.CreationsMusic.com ** DANCE MUSIC/ TOP 40 ** HEY MAMA – FEAT. NICKI MINAJ & AFROJACK RATHER BE – CLEAN BANDIT ALL ABOUT THAT BASS – MEGHAN TRAINOR LIPS ARE MOVIN – MEGHAN TRAINOR TIMES OF YOUR LIVES – PITBULL & NE-YO THINKING OUT LOUD – ED SHEERAN BLANK SPACE– TAYLOR SWIFT BREAK FREE – ARIANA GRANDE BLAME – CALVIN HARRIS TIMBER – PITBULL FEAT. KESHA BURN – ELLIE GOULDING SUMMER – CALVIN HARRIS CHANDELIER – SIA ALL OF ME – JOHN LEGEND COUNTING STARS – ONE REPUBLIC STAY THE NIGHT – ZEDD FEAT HAYLEY WILLIAMS BLURRED LINES – FEAT, T.I. & PHARRELL HAPPY – PHARRELL WILLIAMS CLARITY- ZEDD ROAR –KATY PERRY I NEED YOUR LOVE – ELLIE GOULDING WAKE ME UP – AVICCI RIGHT NOW – RIHANNA I CHOOSE YOU – SARA BAREILLES SAFE AND SOUND – CAPITAL CITIES ALIVE - KREWELLA GET LUCKY – DAFT PHARRELL I LOVE IT – ICONA POP I WILL WAIT – MUMFORD & SONS (RED ROCKS VERSION) HEY HO – THE LUMINEERS LITTLE TALKS – OF MONSTERS AND MEN 1 DON OMAR – DANZA KUDARO FEEL THIS MOMENT – PITBULL FEAT. CHRISTINA AGUILERA DIAMONDS – RIHANNA STAY - RIHANNA HOME – PHILLIP PHILLIPS GONE – PHILLIP PHILLIPS DIE YOUNG – KESHA GIRL ON FIRE –ALICIA KEYS DAYLIGHT – MAROON 5 LOVE SOMEBODY – MAROON 5 DRIVE BY- TRAIN THE A TEAM – ED SHEERAN LOCKED OUT OF HEAVEN – BRUNO MARS DON’T YOU WORRY CHILD – SWEEDISH HOUSE MAFIA SWEET NOTHING – CALVIN HARRIS/FLORENCE WELCH JUST GIVE ME A REASON – PINK FEAT. FUN CALL ME MAYBE – CARLY RAE JEPSEN GLAD YOU CAME – THE WANTED GOTYE – SOMEBODY THAT I USED TO KNOW TITANIUM – DAVID GUETTA & SIA WILD ONES – SIA/FLO-RIDA FEEL SO CLOSE – CALVIN HARRIS TURN ME ON – NIKKI MINAJ MR. -

(2017) Р.1 the Scientific Heritage
No 11 (11) (2017) Р.1 The scientific heritage (Budapest, Hungary) The journal is registered and published in Hungary. The journal publishes scientific studies, reports and reports about achievements in different scientific fields. Journal is published in English, Hungarian, Polish, Russian, Ukrainian, German and French. Articles are accepted each month. Frequency: 12 issues per year. Format - A4 ISSN 9215 — 0365 All articles are reviewed Free access to the electronic version of journal Edition of journal does not carry responsibility for the materials published in a journal. Sending the article to the editorial the author confirms it’s uniqueness and takes full responsibility for possible consequences for breaking copyright laws Chief editor: Biro Krisztian Managing editor: Khavash Bernat Gridchina Olga - Ph.D., Head of the Department of Industrial Management and Logistics (Moscow, Russian Federation) Singula Aleksandra - Professor, Department of Organization and Management at the University of Zagreb (Zagreb, Croatia) Bogdanov Dmitrij - Ph.D., candidate of pedagogical sciences, managing the laboratory (Kiev, Ukraine) Chukurov Valeriy - Doctor of Biological Sciences, Head of the Department of Biochemistry of the Faculty of Physics, Mathematics and Natural Sciences (Minsk, Republic of Belarus) Torok Dezso - Doctor of Chemistry, professor, Head of the Department of Organic Chemistry (Budapest, Hungary) Filipiak Pawel - doctor of political sciences, pro-rector on a management by a property complex and to the public relations (Gdansk, -

Latvijas Filmas Latvijas Simtgadei Latvi- Jas Kino Industrijai Ir Gan Liels Gods, Gan Pamatīgs Izaicinājums
LATVIJAS FILMAS LATVIJAS SIMTGADEI 8 10 12 14 SATURS CONTENT IEVADS 5 DITA RIETUMA INTRODUCTION 6 LINDA PAVĻUTA 7 MĀRA ZĀLĪTE 16 18 20 22 SPĒLFILMAS 8 1906 | 1906 FEATURES 10 BILLE | BILLE 12 HOMO NOVUS | HOMO NOVUS 14 PARADĪZE ‘89 | PARADISE ‘89 16 PUIKA AR SUNI | BOY WITH A DOG 18 VECTĒVS, KAS BĪSTAMĀKS PAR DATORU | GRANDDAD MORE DANGEROUS THAN COMPUTER ANIMĀCIJAS FILMAS 20 JĒKABS, MIMMI UN RUNĀJOŠIE SUŅI | 24 26 28 30 ANIMATION JĒKABS, MIMMI AND TALKING DOGS 22 SAULE BRAUCA DEBESĪS | THE SUN RIDES UP INTO THE SKY DOKUMENTĀLĀS FILMAS 24 ASTOŅAS ZVAIGZNES | EIGHT STARS DOCUMENTARIES 26 BALTIJAS JAUNAIS VILNIS | BALTIC NEW WAVE 28 BALTU CILTIS | BALTIC TRIBES 30 IEVAINOTAIS JĀTNIEKS | THE WOUNDED RIDER 32 KURTS FRIDRIHSONS | KURTS FRIDRIHSONS 34 LUSTRUM | LUSTRUM 36 MĒRIJAS CEĻOJUMS | MĒRIJA’S JOURNEY 32 34 36 38 38 TURPINĀJUMS | CONTINUATION 3 Iespēja īstenot filmu programmu Latvijas filmas Latvijas simtgadei Latvi- jas kino industrijai ir gan liels gods, gan pamatīgs izaicinājums. Nacionālā Kino centra mērķis, uzsākot īstenot šo programmu ar vairāk nekā 7 miljo- niem eiro finansējuma, ir augstvērtīgu, žanriski daudzveidīgu un sabiedriski nozīmīgu filmu izveide, lai aktualizētu Latvijas vēstures, valstiskuma un na- cionālās identitātes tēmas un visplašākajā auditorijā stiprinātu izpratni par Latvijas valsts attīstību. Priecājamies un lepojamies, ka programmas ietvaros līdz 2018. gadam tiks izveidotas 16 pilnmetrāžas filmas – sešas spēlfilmas, divas animācijas filmas un astoņas dokumentālās filmas. Šo filmu tematika ir daudzveidīga un pla- ša – tā aptver gan senus, gan nesenus vēstures posmus, turklāt filmu projek- tu īstenošanā darbojas gan atzīti Latvijas kino klasiķi, gan jaunās paaudzes režisori. Tiksimies pirmizrādēs 2018. gadā! DITA RIETUMA, Nacionālā Kino centra vadītāja The possibility of implementing the film programmeLatvian Films for Latvi- an Centenary is both great honour, as well as a considerable challenge for the Latvian film industry. -

Kaleidoscope Song List (Song List Is Always Subject to Change)
Kaleidoscope Song List (Song List Is Always Subject To Change) Dance Music Ain’t Nobody - Chaka Khan Ain’t No Stoppin’ Us Now - McFadden & Whitehead All About that Bass –Meghan Trainor Bad Romance – Lady Gaga Billionaire – Travis McCoy Blurred Lines- Robin Thicke Boom Boom Pow – Black Eyed Peas Brick house - Commodores Brown-Eyed Girl - Van Morrison California Gurls – Katy Perry Call Me Maybe – Carly Jepsen Can’t Get Next To You - Temptations Car Wash - Rose Royce Celebration - Kool & The Gang Chain Chain Chain – Aretha Franklin Crazy In Love – Beyonce DJ’s Got Us Falling in Love DaButt - E. U. Dancing In The Streets - Martha & The Vandellas Dancing Queen - Abba Don’t Leave Me This Way - Thelma Houston Don’t Stop Believing - Journey Dynamite – Taio Cruz Everybody, Everybody - Black Box (Every Time I Turn Around) Back In Love Again- L.T.D. Finally - Ce Ce Piniston Forget Me Nots - Patrice Rushen Get Down Tonight - KC & The Sunshine Band Get Low - Flo Rida Get Lucky – Daft Punk Get the Girl Back - Hanson Get This Party Started - Pink Give It To Me Baby - Rick James Gonna Make You Sweat - C & C Music Factory Got To Be Real - Cheryl Lynn Happy – Pharrell Williams Hey Ya - Outkast Hit Me With Your Best Shot - Pat Benatar Hot In Here - Nelly Hot Stuff - Donna Summer I Feel Good - James Brown I Got A Feeling – Black Eyed Peas I Heard It Through The Grapevine - Gladys Knight It’s Raining Men - Weather Girls I Will Survive - Gloria Gaynor Into The Mystic – Van Morrison Jump, Jive & Wail - Brian Setzer Orchestra Just Fine – Mary J. -

Tavern TARAS BULBA”
We have only fresh and savory news! September 2012 | №09(108) DON’T MISS: “KORCHMA” BIRTHDAY BULBA September, 13 – 47, Myasnitskaya St. To see more news and photos visit our web site www.tarasbulba.ru e [email protected] U v o k l Project Manager: Yuriy Beloyvan r h a t [email protected] in i i w helpline: 778 3430 an e c ad uisine – m STEVEN SEAGAL In «Korchma» HELPING HAND «Korchma» helps victims of the flood in the Kuban VLADIMIR VYSOTSKY AND THE UKRAINE Kiev roots of nation-wide beloved singer CULINARY ALCHEMISTRY How the Gutsuls cook whyte cheese Wi-Fi available all through KORCHMA 2 | 2 | РубРика РубРика STEVEN SEAGAL: «I LIKE THE UKRAINIAN CUISINE» text: Anastasia Solovey STEVEN SEAGAL - THE AMERICAN ACTOR AND DIRECTOR, MASTER OF MARTIAL ARTS, WORLD-KNOWN THANK TO THE SUSPENSEFUL ACTION FILMS, BEING IN MOSCOW VISITED ON AUGUST, 9 THE RESTAUTRANT OF THE UKRAINIAN CUISINE “KORCHMA TARAS BULBA”.THE UNEXPECTED APPEARANCE OF THE HOLLYWOOD STAR IN THE RESTAURANT CAUSED A FUROR AMONG THE GUESTS. BUT THIS FACT DIDN’T KEEP SEAGAL FROM TASTING WITH PLEASURE THE UKRAINIAN VIAND. «GOOD DAY» As befits to the national tradition, at entrance to the restaurant the guest was welcomed with bread and salt and greeted in the Ukrainian, Russian and English languages. Seagal in his turn loved back, saying “Good day” in Russian. Steven is a frequent guest in our country that is why he is acquainted with our traditions – there was no need to prompt how to act in the following situation: he immediately broke off a piece of loaf and dressed it with salt. -

Pitbull Feat Chris Brown Real Love
16 Elkland Road Melville, NY 11747 631-643-2561 631-643-2563 Fax www.CreationsMusic.com ** DANCE MUSIC/ TOP 40 ** SHUT UP AND DANCE – WALK THE MOON FUN – PITBULL FEAT CHRIS BROWN REAL LOVE – CLEAN BANDIT STYLE – TAYLOR SWIFT SUGAR –MAROON 5 UPTOWN FUNK – BRUNO MARS DEAR FUTURE HUSBAND – MEGHAN TRAINOR SEX ON FIRE – KINGS OF LEON HEY MAMA – FEAT. NICKI MINAJ & AFROJACK SHUT UP AND DANCE – WALK THE MOON RATHER BE – CLEAN BANDIT ALL ABOUT THAT BASS – MEGHAN TRAINOR LIPS ARE MOVIN – MEGHAN TRAINOR TIMES OF YOUR LIVES – PITBULL & NE-YO THINKING OUT LOUD – ED SHEERAN BLANK SPACE– TAYLOR SWIFT BREAK FREE – ARIANA GRANDE BLAME – CALVIN HARRIS TIMBER – PITBULL FEAT. KESHA BURN – ELLIE GOULDING SUMMER – CALVIN HARRIS CHANDELIER – SIA ALL OF ME – JOHN LEGEND COUNTING STARS – ONE REPUBLIC STAY THE NIGHT – ZEDD FEAT HAYLEY WILLIAMS BLURRED LINES – FEAT, T.I. & PHARRELL HAPPY – PHARRELL WILLIAMS CLARITY- ZEDD ROAR –KATY PERRY I NEED YOUR LOVE – ELLIE GOULDING 1 WAKE ME UP – AVICCI RIGHT NOW – RIHANNA I CHOOSE YOU – SARA BAREILLES SAFE AND SOUND – CAPITAL CITIES ALIVE - KREWELLA GET LUCKY – DAFT PHARRELL I LOVE IT – ICONA POP I WILL WAIT – MUMFORD & SONS (RED ROCKS VERSION) HEY HO – THE LUMINEERS LITTLE TALKS – OF MONSTERS AND MEN DON OMAR – DANZA KUDARO FEEL THIS MOMENT – PITBULL FEAT. CHRISTINA AGUILERA DIAMONDS – RIHANNA STAY - RIHANNA HOME – PHILLIP PHILLIPS GONE – PHILLIP PHILLIPS DIE YOUNG – KESHA GIRL ON FIRE –ALICIA KEYS DAYLIGHT – MAROON 5 LOVE SOMEBODY – MAROON 5 DRIVE BY- TRAIN THE A TEAM – ED SHEERAN LOCKED OUT OF HEAVEN – BRUNO MARS DON’T YOU WORRY CHILD – SWEEDISH HOUSE MAFIA SWEET NOTHING – CALVIN HARRIS/FLORENCE WELCH JUST GIVE ME A REASON – PINK FEAT. -

Business Aviation at Riga Airport
Opportunities for Business Aviation October, 2012 Liene Freivalde, Director Aviation Services Department Facts about Riga Airport • RIGA Airport was built in 1974. It was used for domestic flights within USSR • After Latvia regained its independence in 1991 – RIGA Airport became a RIGA International Airport • RIGA International Airport is operated by SJSC RIGA International Airport • The owner of all shares is the Republic of Latvia • Airport occupies the area of 650 ha • Airport has more than 1 050 employees • Net annual turnover was 33 million EUR in 2011 Development stages 1994 - Runway reconstruction 1999 – New arrivals building 2001 – New departure pier with 5 air bridges 2007 – New non-Schengen departure building 2008 – runway prolongation 2013 – 2014 runway and apron reconstruction Airfield reconstruction project will commence soon Project costs more than 95 million EUR 61% Co-financed by EU Cohesion fund 39% Riga International Airport financing Beginning of construction works – Spring 2013 End of construction works – December 2014 Passenger Traffic Growth Passenger Traffic at RIGA Airport has grown significantly in recent years, even during troubled times RIGA ensured double-digit passenger growth! PAX: 2009 +10.2%; 2010 +14.7%: `2011 +10.9 % (Passengers, Millions) 5,1 4,6 4,0 3,6 3,1 2,4 1,8 1,0 0,6 0,6 0,7 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 Source: RIGA Airport Riga Airport regular route map 17 Airlines Serving RIGA Airline Market Share (Carriers by number of passengers served, January – September 2012) Turkish -

Scientific Achievements of Modern Society
SCIENTIFIC ACHIEVEMENTS OF MODERN SOCIETY Abstracts of III International Scientific and Practical Conference Liverpool, United Kingdom 6-8 November 2019 Liverpool, United Kingdom 2019 2 UDC 001.1 BBK 83 The 3rd International scientific and practical conference “Scientific achievements of modern society” (November 6-8, 2019) Cognum Publishing House, Liverpool, United Kingdom. 2019. 549 p. ISBN 978-92-9472-193-8 The recommended citation for this publication is: Ivanov I. Analysis of the phaunistic composition of Ukraine // Scientific achievements of modern society. Abstracts of the 3rd International scientific and practical conference. Cognum Publishing House. Liverpool, United Kingdom. 2019. Pp. 21-27. URL: http://sci-conf.com.ua. Editor Komarytskyy M.L. Ph.D. in Economics, Associate Professor Editorial board prof. Jan Kuchar, CSc. prof. Vaclav Grygar, CSc. doc. PhDr. David Novotny, Ph.D. prof. Vaclav Helus, CSc. doc. PhDr. Zdenek Salac, Ph.D. prof. Vera Winterova, CSc. prof. Ing. Karel Marsalek, M.A., Ph.D. prof. Jiri Cisar, CSc. prof. Ing. Jiri Smolik, M.A., Ph.D. prof. Zuzana Syllova, CSc. prof. Karel Hajek, CSc. prof. Pavel Suchanek, CSc. prof. Alena Svarcova, CSc. prof. Katarzyna Hofmannova, CSc. prof. Marek Jerabek, CSc. prof. Alena Sanderova, CSc. Collection of scientific articles published is the scientific and practical publication, which contains scientific articles of students, graduate students, Candidates and Doctors of Sciences, research workers and practitioners from Europe, Ukraine, Russia and from neighbouring coutries and beyond. The articles contain the study, reflecting the processes and changes in the structure of modern science. The collection of scientific articles is for students, postgraduate students, doctoral candidates, teachers, researchers, practitioners and people interested in the trends of modern science development. -

Scientific Research of the Sco Countries: Synergy and Integration
SCIENTIFIC RESEARCH OF THE SCO COUNTRIES: SYNERGY AND INTEGRATION 上合组织国家的科学研究:协同和一体化 Proceedings of the Date: International Conference September 16 Beijing, China 2020 上合组织国家的科学研究:协同和一体化 国际会议 参与者的英文报告 International Conference “Scientific research of the SCO countries: synergy and integration” Part 1: Participants’ reports in English 2020年9月16日。中国北京 September 16, 2020. Beijing, PRC Proceedings of the International Conference “Scientific research of the SCO countries: synergy and integration”. Part 1 - Reports in English (September 16, 2020. Beijing, PRC) ISBN 978-5-905695-68-2 这些会议文集结合了会议的材料 - 研究论文和科学工作 者的论文报告。 它考察了职业化人格的技术和社会学问题。 一些文章涉及人格职业化研究问题的理论和方法论方法和原 则。 作者对所引用的出版物,事实,数字,引用,统计数据,专 有名称和其他信息的准确性负责 These Conference Proceedings combine materials of the conference – research papers and thesis reports of scientific workers. They examines tecnical and sociological issues of research issues. Some articles deal with theoretical and methodological approaches and principles of research questions of personality professionalization. Authors are responsible for the accuracy of cited publications, facts, figures, quotations, statistics, proper names and other information. ISBN 978-5-905695-68-2 ©Scientific publishing house Infinity, 2020 ©Group of authors, 2020 CONTENTS ECONOMICS 香港的经济和政治前途 Hong Kong's economic and political future Stepanov Nikita Sergeevich................................................................................11 俄罗斯联邦发展国内粮食援助 Development of domestic food aid in the Russian Federation Voronin Boris Alexandrovich, Chupina