Quantum Dots: Theory, Application, Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantum Dots
Quantum Dots www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 1 Outline • Introduction • Quantum Confinement • QD Synthesis – Colloidal Methods – Epitaxial Growth • Applications – Biological – Light Emitters – Additional Applications www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 2 Introduction Definition: • Quantum dots (QD) are nanoparticles/structures that exhibit 3 dimensional quantum confinement, which leads to many unique optical and transport properties. Lin-Wang Wang, National Energy Research Scientific Computing Center at Lawrence Berkeley National Laboratory. <http://www.nersc.gov> GaAs Quantum dot containing just 465 atoms. www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 3 Introduction • Quantum dots are usually regarded as semiconductors by definition. • Similar behavior is observed in some metals. Therefore, in some cases it may be acceptable to speak about metal quantum dots. • Typically, quantum dots are composed of groups II-VI, III-V, and IV-VI materials. • QDs are bandgap tunable by size which means their optical and electrical properties can be engineered to meet specific applications. www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 4 Quantum Confinement Definition: • Quantum Confinement is the spatial confinement of electron-hole pairs (excitons) in one or more dimensions within a material. – 1D confinement: Quantum Wells – 2D confinement: Quantum Wire – 3D confinement: Quantum Dot • Quantum confinement is more prominent in semiconductors because they have an energy gap in their electronic band structure. • Metals do not have a bandgap, so quantum size effects are less prevalent. Quantum confinement is only observed at dimensions below 2 nm. www.nano4me.org © 2018 The Pennsylvania State University Quantum Dots 5 Quantum Confinement • Recall that when atoms are brought together in a bulk material the number of energy states increases substantially to form nearly continuous bands of states. -

Paper 73-3 Has Been Designated As a Distinguished Paper at Display Week 2018
Distinguished Student Paper 73-3 / T. Ji Paper 73-3 has been designated as a Distinguished Paper at Display Week 2018. The full- length version of this paper appears in a Special Section of the Journal of the Society for Information Display (JSID) devoted to Display Week 2018 Distinguished Papers. This Special Section will be freely accessible until December 31, 2018 via: http://onlinelibrary.wiley.com/page/journal/19383657/homepage/display_week_2018.htm Authors that wish to refer to this work are advised to cite the full-length version by referring to its DOI: https://doi.org/10.1002/jsid.640 SID 2018 DIGEST Distinguished Student Paper 73-3 / T. Ji Tingjing Ji- Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Full Color Quantum Dot Light-Emitting Diodes Patterned by Photolithography Technology Tingjing Ji (student), Shuang Jin, Bingwei Chen, Yucong Huang, Zijing Huang, Zinan Chen, Shuming Chen*, Xiaowei Sun Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, PR China, 518055 *Corresponding author: [email protected] Abstract the traditional patterning processes. The QLED device achieved Photolithography is a high resolution and mature patterning maximum electroluminescence intensity of 23 770 cd/m2. [13] technique which has been widely used in semiconductor industry. For display application, a pixel consists of red (R), green (G) and In this work, we use photolithography to fine pattern the QD blue (B) side-by-side sub-pixels, which thereby requires a high layers. Because it is difficult to etch the QD layer, lift-off is used resolution patterning of the light-emission layers. -

Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography
Nanofabrication 2015; 2: 19–26 Research Article Open Access Soma Biswas*, Falko Brinkmann, Michael Hirtz, Harald Fuchs Patterning of Quantum Dots by Dip-Pen and Polymer Pen Nanolithography Abstract: We present a direct way of patterning CdSe/ lithography and photolithography, these direct techniques ZnS quantum dots by dip-pen nanolithography and do not rely on resist layers and multi-step processing, but polymer pen lithography. Mixtures of cholesterol and allow the precise deposition of ink mixtures. Spot sizes in phospholipid 1,2-dioleoyl-sn-glycero-3 phosphocholine commercially widespread inkjet printing and other related serve as biocompatible carrier inks to facilitate the transfer spotting techniques are usually in the range of 50 to 500 of quantum dots from the tips to the surface during µm [5–7], while high resolution approaches (µCP, DPN and lithography. While dip-pen nanolithography of quantum PPL) reach sub-µm features. dots can be used to achieve higher resolution and smaller Microcontact printing utilises a predesigned pattern features (approximately 1 µm), polymer pen poly(dimethylsiloxane) (PDMS) stamp that is first coated lithography is able to address intermediate pattern scales with ink and subsequently pressed onto the surface in the low micrometre range. This allows us to combine manually. A total area up to cm2 can be patterned retaining the advantages of micro contact printing in large area and a lateral resolution of approximately 100 nm [8]. Dip-pen massive parallel patterning, with the added flexibility in nanolithography (Figure 1a) employs an atomic force pattern design inherent in the DPN technique. microscopy tip (AFM) as a quill pen. -

Hyperbolic Metamaterials Based on Quantum-Dot Plasmon-Resonator Nanocomposites
Downloaded from orbit.dtu.dk on: Oct 04, 2021 Hyperbolic metamaterials based on quantum-dot plasmon-resonator nanocomposites. Zhukovsky, Sergei; Ozel, T.; Mutlugun, E.; Gaponik, N.; Eychmuller, A.; Lavrinenko, Andrei; Demir, H. V.; Gaponenko, S. V. Published in: Optics Express Link to article, DOI: 10.1364/OE.22.018290 Publication date: 2014 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Zhukovsky, S., Ozel, T., Mutlugun, E., Gaponik, N., Eychmuller, A., Lavrinenko, A., Demir, H. V., & Gaponenko, S. V. (2014). Hyperbolic metamaterials based on quantum-dot plasmon-resonator nanocomposites. Optics Express, 22(15), 18290-18298. https://doi.org/10.1364/OE.22.018290 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Hyperbolic metamaterials based on quantum-dot plasmon-resonator nanocomposites 1, 2 2,3 4 S. V. Zhukovsky, ∗ T. Ozel, E. Mutlugun, N. Gaponik, A. -

Quantum Dot and Electron Acceptor Nano-Heterojunction For
www.nature.com/scientificreports OPEN Quantum dot and electron acceptor nano‑heterojunction for photo‑induced capacitive charge‑transfer Onuralp Karatum1, Guncem Ozgun Eren2, Rustamzhon Melikov1, Asim Onal3, Cleva W. Ow‑Yang4,5, Mehmet Sahin6 & Sedat Nizamoglu1,2,3* Capacitive charge transfer at the electrode/electrolyte interface is a biocompatible mechanism for the stimulation of neurons. Although quantum dots showed their potential for photostimulation device architectures, dominant photoelectrochemical charge transfer combined with heavy‑metal content in such architectures hinders their safe use. In this study, we demonstrate heavy‑metal‑free quantum dot‑based nano‑heterojunction devices that generate capacitive photoresponse. For that, we formed a novel form of nano‑heterojunctions using type‑II InP/ZnO/ZnS core/shell/shell quantum dot as the donor and a fullerene derivative of PCBM as the electron acceptor. The reduced electron–hole wavefunction overlap of 0.52 due to type‑II band alignment of the quantum dot and the passivation of the trap states indicated by the high photoluminescence quantum yield of 70% led to the domination of photoinduced capacitive charge transfer at an optimum donor–acceptor ratio. This study paves the way toward safe and efcient nanoengineered quantum dot‑based next‑generation photostimulation devices. Neural interfaces that can supply electrical current to the cells and tissues play a central role in the understanding of the nervous system. Proper design and engineering of such biointerfaces enables the extracellular modulation of the neural activity, which leads to possible treatments of neurological diseases like retinal degeneration, hearing loss, diabetes, Parkinson and Alzheimer1–3. Light-activated interfaces provide a wireless and non-genetic way to modulate neurons with high spatiotemporal resolution, which make them a promising alternative to wired and surgically more invasive electrical stimulation electrodes4,5. -

1.07 Quantum Dots: Theory N Vukmirovic´ and L-W Wang, Lawrence Berkeley National Laboratory, Berkeley, CA, USA
1.07 Quantum Dots: Theory N Vukmirovic´ and L-W Wang, Lawrence Berkeley National Laboratory, Berkeley, CA, USA ª 2011 Elsevier B.V. All rights reserved. 1.07.1 Introduction 189 1.07.2 Single-Particle Methods 190 1.07.2.1 Density Functional Theory 191 1.07.2.2 Empirical Pseudopotential Method 193 1.07.2.3 Tight-Binding Methods 194 1.07.2.4 k ? p Method 195 1.07.2.5 The Effect of Strain 198 1.07.3 Many-Body Approaches 201 1.07.3.1 Time-Dependent DFT 201 1.07.3.2 Configuration Interaction Method 202 1.07.3.3 GW and BSE Approach 203 1.07.3.4 Quantum Monte Carlo Methods 204 1.07.4 Application to Different Physical Effects: Some Examples 205 1.07.4.1 Electron and Hole Wave Functions 205 1.07.4.2 Intraband Optical Processes in Embedded Quantum Dots 206 1.07.4.3 Size Dependence of the Band Gap in Colloidal Quantum Dots 208 1.07.4.4 Excitons 209 1.07.4.5 Auger Effects 210 1.07.4.6 Electron–Phonon Interaction 212 1.07.5 Conclusions 213 References 213 1.07.1 Introduction laterally by electrostatic gates or vertically by etch- ing techniques [1,2]. The properties of this type of Since the early 1980s, remarkable progress in technology quantum dots, sometimes termed as electrostatic has been made, enabling the production of nanometer- quantum dots, can be controlled by changing the sized semiconductor structures. This is the length scale applied potential at gates, the choice of the geometry where the laws of quantum mechanics rule and a range of gates, or external magnetic field. -

Electrodynamic Modeling of Quantum Dot Luminescence in Plasmonic Metamaterials † ‡ † ‡ ‡ § Ming Fang,*, , Zhixiang Huang,*, Thomas Koschny, and Costas M
Article pubs.acs.org/journal/apchd5 Electrodynamic Modeling of Quantum Dot Luminescence in Plasmonic Metamaterials † ‡ † ‡ ‡ § Ming Fang,*, , Zhixiang Huang,*, Thomas Koschny, and Costas M. Soukoulis , † Key Laboratory of Intelligent Computing and Signal Processing, Ministry of Education, Anhui University, Hefei 230001, China ‡ Ames Laboratory and Department of Physics and Astronomy, Iowa State University, Ames, Iowa 50011, United States § Institute of Electronic Structure and Laser, FORTH, 71110 Heraklion, Crete, Greece ABSTRACT: A self-consistent approach is proposed to simulate a coupled system of quantum dots (QDs) and metallic metamaterials. Using a four-level atomic system, an artificial source is introduced to simulate the spontaneous emission process in the QDs. We numerically show that the metamaterials can lead to multifold enhancement and spectral narrowing of photoluminescence from QDs. These results are consistent with recent experimental studies. The proposed method represents an essential step for developing and understanding a metamaterial system with gain medium inclusions. KEYWORDS: photoluminescence, plasmonics, metamaterials, quantum dots, finite-different time-domain, spontaneous emission he fields of plasmonic metamaterials have made device design based on quantum electrodynamics. In an active − T spectacular experimental progress in recent years.1 3 medium, the electromagnetic field is treated classically, whereas The metal-based metamaterial losses at optical frequencies atoms are treated quantum mechanically. According to the are unavoidable. Therefore, control of conductor losses is a key current understanding, the interaction of electromagnetic fields challenge in the development of metamaterial technologies. with an active medium can be modeled by a classical harmonic These losses hamper the development of optical cloaking oscillator model and the rate equations of atomic population devices and negative index media. -
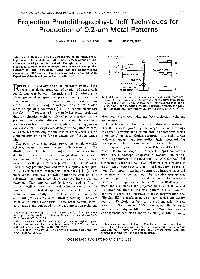
Projection Photolithography-Liftoff Techniques for Production of 0.2-Pm Metal Patterns
IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. ED-28, NO. 11, NOVEMBER 1981 1375 Projection Photolithography-Liftoff Techniques for Production of 0.2-pm Metal Patterns MARK D. FEUER AND DANIEL E. PROBER, MEMBER, IEEE GLASS FILTER Abstract-A technique whichallows the useof projection photo- \ yPHOTORESIST lithography with the photoresist liftoff process, for fabrication of sub- micrometer metal patterns, is described. Through-the-substrate (back- \II a projection) exposure of the photoresist produces the undercut profiles necessary forliftoff processing. Metal lines andsuperconducting microbridges of 0.2-pm width have been fabricated with this technique. I Experimental details and process limits are discussed. II OBJECTIVE IMMERSION OIL ECENT DEVELOPMENTS inmicrolithography have SUBSTRATE - (d) R made possible the production ofa variety of devices with PHOTORESIST %- I-l submicrometer (submicron) dimensions [ 11 , [2] , offering the (a) advantages of higherspeed and packing density.For many Fig. 1. Schematicdiagram of back-projectionand metal-liftoff pro- Josephson-effect devices in particular, submicron dimensions cedure. (a) Exposure system, employing a Zeiss optical microscope. Image of the mask is projected through the substrate, which is shown are essential for achieving optimalperformance over awide in sideview. (b) Schematic contours of constant exposure intensity. range of operatingconditions [3] . Forsubmicron pattern (c) After photoresist development and metallization. (d) After liftoff. transfer, liftoff processing [ 11 generally -

Quantum Computation with Two-Dimensional Graphene
Quantum computation with two-dimensional graphene quantum dots* Jason Lee(李杰森), Zhi-Bing Li(李志兵), and Dao-Xin Yao (姚道新)†† State Key Laboratory of Optoelectronic Materials and Technologies, School of Physics and Engineering, Sun Yat-sen University, Guangzhou 510275, China Keywords: graphene, quantum dot, quantum computation, Kagome lattice PACS: 73.22.Pr, 73.21.La, 73.22.–f, 74.25.Jb Abstract We study an array of graphene nano sheets that form a two-dimensional S = 1/2 Kagome spin lattice used for quantum computation. The edge states of the graphene nano sheets are used to form quantum dots to confine electrons and perform the computation. We propose two schemes of bang-bang control to combat decoherence and realize gate operations on this array of quantum dots. It is shown that both schemes contain a great amount of information for quantum computation. The corresponding gate operations are also proposed. 1. Introduction There has been increasing interest in grapheme since its discovery. [1−3] It has shown excellent electronic[4,5] and mechanical [6,7] properties and is also a promising candidate for biosensors.[8,9] Before this amazing discovery, Wallace had studied the band structure of graphite and found a linear dispersion around the Dirac point in the Brillouin zone. [10]Much research has been done on this linear dispersion and in particular on the transport properties of graphene. [11−13] Nakada and Fujita studied the edge state and the nano size effect of graphene, and found that the charge can be localized in the zigzag edge[14] to form quantum dots (QDs). -

And Nanolithography Techniques and Their Applications
Review on Micro- and Nanolithography Techniques and their Applications Alongkorn Pimpin* and Werayut Srituravanich** Department of Mechanical Engineering, Faculty of Engineering, Chulalongkorn University, Pathumwan, Bangkok 10330, Thailand E-mail: [email protected]*, [email protected]** Abstract. This article reviews major micro- and nanolithography techniques and their applications from commercial micro devices to emerging applications in nanoscale science and engineering. Micro- and nanolithography has been the key technology in manufacturing of integrated circuits and microchips in the semiconductor industry. Such a technology is also sparking revolutionizing advancements in nanotechnology. The lithography techniques including photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography and scanning probe lithography are discussed. Furthermore, their applications are summarized into four major areas: electronics and microsystems, medical and biotech, optics and photonics, and environment and energy harvesting. Keywords: Nanolithography, photolithography, electron beam lithography, focused ion beam lithography, soft lithography, nanoimprint lithography, scanning probe lithography, dip-pen lithography, microsystems, MEMS, nanoscience, nanotechnology, nano-engineering. ENGINEERING JOURNAL Volume 16 Issue 1 Received 18 August 2011 Accepted 8 November Published 1 January 2012 Online at http://www.engj.org DOI:10.4186/ej.2012.16.1.37 DOI:10.4186/ej.2012.16.1.37 1. Introduction For decades, micro- and nanolithography technology has been contributed to the manufacturing of integrated circuits (ICs) and microchips. This advance in the semiconductor and IC industry has led to a new paradigm of the information revolution via computers and the internet. Micro- and nanolithography is the technology that is used to create patterns with a feature size ranging from a few nanometers up to tens of millimeters. -

Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems
micromachines Review Techniques and Considerations in the Microfabrication of Parylene C Microelectromechanical Systems Jessica Ortigoza-Diaz 1, Kee Scholten 1 ID , Christopher Larson 1 ID , Angelica Cobo 1, Trevor Hudson 1, James Yoo 1 ID , Alex Baldwin 1 ID , Ahuva Weltman Hirschberg 1 and Ellis Meng 1,2,* 1 Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA; [email protected] (J.O.-D.); [email protected] (K.S.); [email protected] (C.L.); [email protected] (A.C.); [email protected] (T.H.); [email protected] (J.Y.); [email protected] (A.B.); [email protected] (A.W.H.) 2 Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA 90089, USA * Correspondence: [email protected]; Tel.: +1-213-740-6952 Received: 31 July 2018; Accepted: 18 August 2018; Published: 22 August 2018 Abstract: Parylene C is a promising material for constructing flexible, biocompatible and corrosion- resistant microelectromechanical systems (MEMS) devices. Historically, Parylene C has been employed as an encapsulation material for medical implants, such as stents and pacemakers, due to its strong barrier properties and biocompatibility. In the past few decades, the adaptation of planar microfabrication processes to thin film Parylene C has encouraged its use as an insulator, structural and substrate material for MEMS and other microelectronic devices. However, Parylene C presents unique challenges during microfabrication and during use with liquids, especially for flexible, thin film electronic devices. In particular, the flexibility and low thermal budget of Parylene C require modification of the fabrication techniques inherited from silicon MEMS, and poor adhesion at Parylene-Parylene and Parylene-metal interfaces causes device failure under prolonged use in wet environments. -

Fundimentals of Photolithography
FUNDIMENTALS OF PHOTOLITHOGRAPHY One of the most widely used methods for creating nanoscale circuit components is Photolithography. The word lithography is derived from the Greek words lithos (stone) and graphein (to write) and finds its roots in a process invented by Aloys Senefelder in 1796. By treating a piece of limestone with certain chemicals, Senefelder was then able to transfer an image carved into the stone onto a piece of paper. This was done by coating certain parts of the porous limestone with a water repellant substance. When ink was applied to the stone it would only adhere to the untreated hydrophilic areas, and hence the image carved into the rock could be transferred repeatedly onto paper. Senefelder's technique is still used in some artistry applications today. As time progressed and technology improved, lithography methods evolved. In the 1820's a French scientist by the name of Nicephore Niepce developed the first photoresist, a component fundamental to photolithography. A photoresist is a substance that undergoes a chemical reaction when it is eXposed to light. Niepce's photoresist was a material known as Bitumen of Judea, a kind of naturally occurring asphalt. A sheet of stone, metal, or glass was coated with a thin layer of this bitumen, which became less soluble where it was eXposed to light. Areas that were uneXposed could then be removed using a solvent, and the resultant exposed areas of the sheet were etched using a chemical bath. After the remaining photoresist was removed, the sheet could then be used as a printing plate. Photolithography today is in many ways similar to the original process invented by Niepce.