A Roadmap of the Human Placenta and Consensus for Tissue and Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Histopathological Analysis of Patients with Abruptio
HISTOPATHOLOGICAL ANALYSIS OF PATIENTS WITH ABRUPTIO PLACENTA IN A TERTIARY HEALTH CARE CENTRE Dissertation submitted in Partial fulfillment of the regulations required for the award of M.D. Degree in PATHOLOGY - BRANCH III THE TAMILNADU DR.M.G.R.MEDICAL UNIVERSITY CHENNAI MAY 2019 DECLARATION I hereby declare that the dissertation entitled “HISTOPATHOLOGICAL ANALYSIS OF PATIENTS WITH ABRUPTIO PLACENTA IN A TERTIARY HEALTH CARE CENTRE” is a bonafide research work done by me in the Department of Pathology, Coimbatore Medical College during the period from JANUARY 2017 TO JUNE 2018 under the guidance and supervision of Dr.G.S.THIRIVENI BALAJJI, M.D, Associate Professor, Department of Pathology, Coimbatore Medical College. This dissertation is submitted to The Tamilnadu Dr.MGR Medical University, Chennai towards the partial fulfillment of the requirement for the award of M.D., Degree (Branch III) in Pathology. I have not submitted this dissertation on any previous occasion to any University for the award of any Degree. Place : Coimbatore Dr. A. PETER SAMIDOSS Date : CERTIFICATE This is to certify that dissertation entitled “HISTOPATHOLOGICAL ANALYSIS OF PATIENTS WITH ABRUPTIO PLACENTA IN A TERTIARY HEALTH CARE CENTRE” is a bonafide work done by Dr. A. PETER SAMIDOSS, a postgraduate student in the Department of Pathology, Coimbatore Medical College, Coimbatore under guidance and supervision of Dr.G.S.THIRIVENI BALAJJI, M.D, Associate Professor, Department of Pathology, Coimbatore Medical College, Coimbatore in partial fulfillment of the regulations of the Tamil Nadu Dr. M. G. R. Medical University, Chennai towards the award of M.D. Degree (Branch III) in Pathology. Guide Head of the Department Dr.G.S.THIRIVENI BALAJJI, M.D, Prof. -

Preeclampsia a Deficiency of Placental IL-10 In
A Deficiency of Placental IL-10 in Preeclampsia A. Hennessy, H. L. Pilmore, L. A. Simmons and D. M. Painter This information is current as of October 4, 2021. J Immunol 1999; 163:3491-3495; ; http://www.jimmunol.org/content/163/6/3491 References This article cites 23 articles, 8 of which you can access for free at: Downloaded from http://www.jimmunol.org/content/163/6/3491.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision http://www.jimmunol.org/ • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: by guest on October 4, 2021 http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1999 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. A Deficiency of Placental IL-10 in Preeclampsia A. Hennessy,1 H. L. Pilmore, L. A. Simmons, and D. M. Painter Accommodation of the fetoplacental unit in human pregnancy requires maternal immune tolerance to this “semiallograft”. Local antiplacental immunity is modified by synthesis of uncommon histocompatibility Ags (e.g., HLA-G), growth factors, and cytokines by the placenta. -

The Therapeutic Potential, Challenges and Future Clinical Directions of Stem Cells from the Wharton’S Jelly of the Human Umbilical Cord
Stem Cell Rev and Rep (2013) 9:226–240 DOI 10.1007/s12015-012-9418-z The Therapeutic Potential, Challenges and Future Clinical Directions of Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord Ariff Bongso & Chui-Yee Fong Published online: 12 December 2012 # Springer Science+Business Media New York 2012 Abstract Mesenchymal stem cells (MSCs) from bone mar- attractive autologous or allogeneic agents for the treatment of row, adult organs and fetuses face the disadvantages of inva- malignant and non-malignant hematopoietic and non- sive isolation, limited cell numbers and ethical constraints hematopoietic diseases. This review critically evaluates their while embryonic stem cells (ESCs) and induced pluripotent therapeutic value, the challenges and future directions for their stem cells (iPSCs) face the clinical hurdles of potential immu- clinical application. norejection and tumorigenesis respectively. These challenges have prompted interest in the study and evaluation of stem Keywords Standardization of derivation protocols . cells from birth-associated tissues. The umbilical cord (UC) Properties and applications of Wharton’s jelly stem cells . has been the most popular. Hematopoietic stem cells (HSCs) Umbilical cord compartments harvested from cord blood have been successfully used for the treatment of hematopoietic diseases. Stem cell populations have also been reported in other compartments of the UC Introduction viz., amnion, subamnion, perivascular region, Wharton’sjelly, umbilical blood vessel adventia and endothelium. Differences Various types of stem cells have been isolated to date in the in stemness characteristics between compartments have been human from a variety of tissues including preimplantation reported and hence derivation protocols using whole UC embryos, fetuses, birth-associated tissues and adult organs. -

Neonate Germinal Stage Blastocyst Embryonic Disk Trophoblast Umbilical Cord Placenta Embryonic Stage Cephalocaudal Proximodistal
neonate umbilical cord Chapter 3 Chapter 3 germinal stage placenta Chapter 3 Chapter 3 blastocyst embryonic stage Chapter 3 Chapter 3 embryonic disk cephalocaudal Chapter 3 Chapter 3 trophoblast proximodistal Chapter 3 Chapter 3 A tube that connects the fetus to the placenta. A newborn baby. Chapter 3 Chapter 3 An organ connected to the uterine wall and to the fetus by the umbilical cord. The placenta The period of development between conception serves as a filter between mother and fetus for and the implantation of the embryo. the exchange of nutrients and wastes. Chapter 3 Chapter 3 The stage of prenatal development that lasts A stage within the germinal period of prenatal from implantation through the eighth week of development in which the zygote has the form pregnancy; it is characterized by the of a sphere of cells surrounding a cavity of fluid. development of the major organ systems. Chapter 3 Chapter 3 The platelike inner part of the blastocyst that From head to tail. differentiates into the ectoderm, mesoderm, and endoderm of the embryo. Chapter 3 Chapter 3 The outer part of the blastocyst from which the From the inner part (or axis) of the body amniotic sac, placenta, and umbilical cord outward. develop. Chapter 3 Chapter 3 ectoderm amniotic sac Chapter 3 Chapter 3 neural tube amniotic fluid Chapter 3 Chapter 3 endoderm fetal stage Chapter 3 Chapter 3 mesoderm stillbirth Chapter 3 Chapter 3 androgens teratogens Chapter 3 Chapter 3 The outermost cell layer of the newly formed The sac containing the fetus. embryo from which the skin and nervous system develop. -

Hyperactivated Wnt-Β-Catenin Signaling in the Absence of Sfrp1 and Sfrp5 Disrupts Trophoblast Differentiation Through Repressio
Bao et al. BMC Biology (2020) 18:151 https://doi.org/10.1186/s12915-020-00883-4 RESEARCH ARTICLE Open Access Hyperactivated Wnt-β-catenin signaling in the absence of sFRP1 and sFRP5 disrupts trophoblast differentiation through repression of Ascl2 Haili Bao1,2,3†, Dong Liu2†, Yingchun Xu2, Yang Sun2, Change Mu2, Yongqin Yu2, Chunping Wang2, Qian Han2, Sanmei Liu2, Han Cai1,2, Fan Liu2, Shuangbo Kong1,2, Wenbo Deng1,2, Bin Cao1,2, Haibin Wang1,2*, Qiang Wang3,4* and Jinhua Lu1,2* Abstract Background: Wnt signaling is a critical determinant for the maintenance and differentiation of stem/progenitor cells, including trophoblast stem cells during placental development. Hyperactivation of Wnt signaling has been shown to be associated with human trophoblast diseases. However, little is known about the impact and underlying mechanisms of excessive Wnt signaling during placental trophoblast development. Results: In the present work, we observed that two inhibitors of Wnt signaling, secreted frizzled-related proteins 1 and 5 (Sfrp1 and Sfrp5), are highly expressed in the extraembryonic trophoblast suggesting possible roles in early placental development. Sfrp1 and Sfrp5 double knockout mice exhibited disturbed trophoblast differentiation in the placental ectoplacental cone (EPC), which contains the precursors of trophoblast giant cells (TGCs) and spongiotrophoblast cells. In addition, we employed mouse models expressing a truncated β-catenin with exon 3 deletion globally and trophoblast-specifically, as well as trophoblast stem cell lines, and unraveled that hyperactivation of canonical Wnt pathway exhausted the trophoblast precursor cells in the EPC, resulting in the overabundance of giant cells at the expense of spongiotrophoblast cells. -

Human Pluripotent Stem Cells As a Model of Trophoblast Differentiation in Both Normal Development and Disease
Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease Mariko Horiia,b,1, Yingchun Lia,b,1, Anna K. Wakelanda,b,1, Donald P. Pizzoa, Katharine K. Nelsona,b, Karen Sabatinib,c, Louise Chang Laurentb,c, Ying Liud,e,f, and Mana M. Parasta,b,2 aDepartment of Pathology, University of California, San Diego, La Jolla, CA 92093; bSanford Consortium for Regenerative Medicine, University of California, San Diego, La Jolla, CA 92093; cDepartment of Reproductive Medicine, University of California, San Diego, La Jolla, CA 92093; dDepartment of Neurosurgery, Center for Stem Cell and Regenerative Medicine, University of Texas Health Sciences Center, Houston, TX 77030; eThe Senator Lloyd and B. A. Bentsen Center for Stroke Research, University of Texas Health Sciences Center, Houston, TX 77030; and fThe Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Sciences Center, Houston, TX 77030 Edited by R. Michael Roberts, University of Missouri–Columbia, Columbia, MO, and approved May 25, 2016 (received for review March 24, 2016) Trophoblast is the primary epithelial cell type in the placenta, a Elf5 (Ets domain transcription factor) and Eomes (Eomeso- transient organ required for proper fetal growth and develop- dermin), also have been shown to be required for maintenance of ment. Different trophoblast subtypes are responsible for gas/nutrient the TSC fate in the mouse (8, 9). exchange (syncytiotrophoblasts, STBs) and invasion and maternal Significantly less is known about TE specification and the TSC vascular remodeling (extravillous trophoblasts, EVTs). Studies of niche in the human embryo (10, 11). -

Messages from the Placentae Across Multiple Species a 50 Years
Placenta 84 (2019) 14–27 Contents lists available at ScienceDirect Placenta journal homepage: www.elsevier.com/locate/placenta Messages from the placentae across multiple species: A 50 years exploration T Hiroaki Soma Saitama Medical University, Japan ARTICLE INFO ABSTRACT Keywords: This review explores eight aspects of placentation in multiple mammalian. Gestational trophoblastic disease 1) Specialities of gestational trophoblastic disease. SUA(Single umbilical artery) 2) Clinical significance of single umbilical artery (SUA) syndrome. DIC(Disseminated intravascular coagulation) in 3) Pulmonary trophoblast embolism in pregnant chinchillas and DIC in pregnant giant panda. giant panda 4) Genetics status and placental behaviors during Japanese serow and related antelopes. Placentation in Japanese serow 5) Specific living style and placentation of the Sloth and Proboscis monkey. Hydatidiform mole in chimpanzee Placentation in different living elephant 6) Similarities of placental structures between human and great apes. Manatee and hyrax 7) Similarities of placental forms in elephants, manatees and rock hyrax with different living styles. Specific placental findings of Himalayan people 8) Specialities of placental pathology in Himalayan mountain people. Conclusions: It was taught that every mammalian species held on placental forms applied to different environ- mental life for their infants, even though their gestational lengths were different. 1. Introduction of effective chemotherapeutic agents. In 1959, I was fortunate tore- ceive an invitation from Prof. Kurt Benirschke at the Boston Lying-in Last October, Scientific American published a special issue about a Hospital. Before that, I had written to Prof. Arthur T. Hertig, Chairman baby's first organ, the placenta [1]. It is full of surprises and amazing of Pathology, Harvard Medical School, asking to study human tropho- science. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -
Glossary of Common MCH Terms and Acronyms
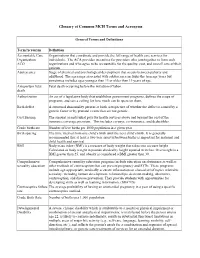
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

Cell Fate in the Early Mouse Embryo: Sorting out the Influence of Developmental History on Lineage Choice
Reproductive BioMedicine Online (2011) 22, 521– 524 www.sciencedirect.com www.rbmonline.com COMMENTARY Cell fate in the early mouse embryo: sorting out the influence of developmental history on lineage choice Samantha A Morris Wellcome Trust/Cancer Research UK Gurdon Institute, University of Cambridge, Tennis Court Road, Cambridge CB2 1QR, UK; University of Cambridge, Department of Physiology, Development and Neurobiology, Downing Street, Cambridge CB2 3DY, UK E-mail address: [email protected]. Abstract In early mouse embryos the first cell-fate decision segregates two cell populations: the outer trophectoderm (TE) and inner cell mass (ICM). Cells are primarily directed to the ICM in two waves of asymmetric division at the 8–16-cell and 16–32-cell stage transition – the first and second waves, respectively. The ICM then diverges to become epiblast (EPI) which will generate the embryo/fetus and extra-embryonic primitive endoderm (PE). Two recent studies have aimed to address the developmental origins of these lineages. Morris et al. (2010) found that first-wave-internalized cells mainly generate EPI, whereas later internalized cells pro- vide PE. This trend was not reflected in an independent study (Yamanaka et al., 2010). From direct comparison of both datasets, it becomes clear that the key difference lies in the proportions of cells internalized in the two waves, impacting greatly upon fate. When the majority of ICM is derived from only the first wave, both EPI and PE must differentiate from the available cells and no pattern is observed. Frequently though, closer parity exists between cells dividing asymmetrically in the first and second waves, revealing the influence of developmental history upon fate. -

Efficient Production of Trophoblast Lineage Cells from Human Induced
Laboratory Investigation (2017) 97, 1188–1200 © 2017 USCAP, Inc All rights reserved 0023-6837/17 Efficient production of trophoblast lineage cells from human induced pluripotent stem cells Junya Kojima1,2, Atsushi Fukuda1, Hayato Taira1, Tomoyuki Kawasaki1, Hiroe Ito2, Naoaki Kuji2, Keiichi Isaka2, Akihiro Umezawa1 and Hidenori Akutsu1,3 Human induced pluripotent stem cells (hiPSCs) are potentially useful in both clinical applications and basic biological research. hiPSCs can differentiate into extra-embryonic cells in the presence of BMP4. However, the differentiation potential of hiPSCs can be affected by culture conditions or genetic variation. In this study, we investigated the effect of various BMP4 concentrations on the expression states of trophoblast markers and the optimal conditions for trophoblast induction. A high-fidelity gene expression assay using hiPSC lines showed that the expression levels of various trophoblast marker genes, such as KRT7, GCM1, CGB, and HLA-G, were upregulated by BMP4 in a dose-dependent manner in all types of hiPSCs used in this study. Treatment with high doses of BMP4 for prolonged periods increased the ratio of cells with trophoblast markers irrespective of the presence of bFGF. We found that the expression states of major pluripotency- and differentiation-related protein-coding genes in BMP4-treated cells depended on culture conditions rather than donor cell types. However, miRNA expression states were affected by donor cell types rather than BMP4 dose. Furthermore, the effect of the presence of bFGF on differentiation potential of KRT7-positive cells differed among iPSC types. Mechanistically, chromatin states around KRT7 promoter regions were comparable among the iPSC types used in this study, indicating that hiPSC chromatin state at these regions is not a parameter for cytotrophoblast differentiation potential.