Guidelines for Minimum Frequency of Antepartum Nonstress Testing and Ultrasound Surveillance WW.04.04A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Perinatal Care, 8Th Ed., Pp. 48-49, 198-205 ACOG Practice Bulletin, No 145, July 2014, Reaffirmed 2016 JOGNN, No
Guidelines for Perinatal Care, 8th ed., pp. 48-49, 198-205 ACOG Practice Bulletin, No 145, July 2014, Reaffirmed 2016 JOGNN, No. 44, pp. 683-686, 2015 Terminology and interpretation of electronic fetal monitoring tracings as defined by National Institute of Child Health and Development (NICHD) Maternal Health Competencies - Fetal Assessment & Antenatal Fetal Surveillance Fetal Heart Tones (Beginning at 10 Weeks Gestation with hand-held Doppler) 1. Review provider or standing order 2. Explain procedure and obtains patient's verbal consent 3. Perform hand hygiene prior to engaging with patient 4. Assist patient into semi-recumbent position, expose abdomen 5. Locate the point of loudest fetal heart tones and a. Palpate maternal pulse b. Utilize pulse oximetry (placed on maternal index finger) 6. Monitor maternal pulse and count fetal heart rate for 1 full minute 7. Record findings 8. Demonstrate knowledge of fetal heart rate ranges: a. Normal = 110-160 bpm b. Bradycardia = < 110 bpm c. Tachycardia = > 160 bpm 9. Demonstrate knowledge of criteria for notifying provider of concerns/findings before, during, or after assessment (per clinical policies) Fundal Height (Beginning at 12 to 14 Weeks gestation) 1. Review provider or standing order 2. Explain procedure and obtain patient's verbal consent 3. Perform hand hygiene prior to engaging with patient 4. Assist patient to semi-recumbent position, expose abdomen 5. Ensure the abdomen is soft and palpates with 2 hands to locate the uterine fundus 6. Measure from top of fundus to top of symphysis pubis using a pliable, non-elastic tape measure, keeping the tape measure in contact with the skin, and measuring along the longitudinal axis without correcting to the abdominal midline 7. -

The Empire Plan SEPTEMBER 2018 REPORTING ON
The Empire Plan SEPTEMBER 2018 REPORTING ON PRENATAL CARE Every baby deserves a healthy beginning and you can take steps before your baby is even born to help ensure a great start for your infant. That’s why The Empire Plan offers mother and baby the coverage you need. When your primary coverage is The Empire Plan, the Empire Plan Future Moms Program provides you with special services. For Empire Plan enrollees and for their enrolled dependents, COBRA enrollees with their Empire Plan benefits and Young Adult Option enrollees TABLE OF CONTENTS Five Important Steps ........................................ 2 Feeding Your Baby ...........................................11 Take Action to Be Healthy; Breastfeeding and Your Early Pregnancy ................................................. 4 Empire Plan Benefits .......................................12 Prenatal Testing ................................................. 5 Choosing Your Baby’s Doctor; New Parents ......................................................13 Future Moms Program ......................................7 Extended Care: Medical Case High Risk Pregnancy Program; Management; Questions & Answers ...........14 Exercise During Pregnancy ............................ 8 Postpartum Depression .................................. 17 Your Healthy Diet During Pregnancy; Medications and Pregnancy ........................... 9 Health Care Spending Account ....................19 Skincare Products to Avoid; Resources ..........................................................20 Childbirth Education -

OBGYN-Study-Guide-1.Pdf
OBSTETRICS PREGNANCY Physiology of Pregnancy: • CO input increases 30-50% (max 20-24 weeks) (mostly due to increase in stroke volume) • SVR anD arterial bp Decreases (likely due to increase in progesterone) o decrease in systolic blood pressure of 5 to 10 mm Hg and in diastolic blood pressure of 10 to 15 mm Hg that nadirs at week 24. • Increase tiDal volume 30-40% and total lung capacity decrease by 5% due to diaphragm • IncreaseD reD blooD cell mass • GI: nausea – due to elevations in estrogen, progesterone, hCG (resolve by 14-16 weeks) • Stomach – prolonged gastric emptying times and decreased GE sphincter tone à reflux • Kidneys increase in size anD ureters dilate during pregnancy à increaseD pyelonephritis • GFR increases by 50% in early pregnancy anD is maintaineD, RAAS increases = increase alDosterone, but no increaseD soDium bc GFR is also increaseD • RBC volume increases by 20-30%, plasma volume increases by 50% à decreased crit (dilutional anemia) • Labor can cause WBC to rise over 20 million • Pregnancy = hypercoagulable state (increase in fibrinogen anD factors VII-X); clotting and bleeding times do not change • Pregnancy = hyperestrogenic state • hCG double 48 hours during early pregnancy and reach peak at 10-12 weeks, decline to reach stead stage after week 15 • placenta produces hCG which maintains corpus luteum in early pregnancy • corpus luteum produces progesterone which maintains enDometrium • increaseD prolactin during pregnancy • elevation in T3 and T4, slight Decrease in TSH early on, but overall euthyroiD state • linea nigra, perineum, anD face skin (melasma) changes • increase carpal tunnel (median nerve compression) • increased caloric need 300cal/day during pregnancy and 500 during breastfeeding • shoulD gain 20-30 lb • increaseD caloric requirements: protein, iron, folate, calcium, other vitamins anD minerals Testing: In a patient with irregular menstrual cycles or unknown date of last menstruation, the last Date of intercourse shoulD be useD as the marker for repeating a urine pregnancy test. -

Antepartum Fetal Heart Rate Testing
Antepartum Fetal Surveillance Dr. M. Mokhtari Associate Professor of OB GYN Primary Goal The primary goal is to identify fetuses at risk of intrauterine death or neonatal complications and intervene (often by delivery) to prevent these adverse outcomes, if possible. NORMAL FETAL MOVEMENT Sonographically fetal activity can be noted as early as 7 to 8 weeks of gestation. Maternal perception of fetal movement begins around 16 to 20 weeks of gestation. The mother's first perception of fetal movement, termed "quickening," is often described as a gentle flutter . Fetal movement increases throughout day, with peak activity late at night. Kick counts ●Perception of least 10 fetal movements (FMs) over up to two hours when the mother is at rest and focused on counting ("count to 10" method) . Patients are instructed to contact within 12 hours for further evaluation if they perceive a significant and persistent reduction in fetal movement and never to wait longer than two hours if there is absent. DIFFERENTIAL DIAGNOSIS Transient decreases in fetal activity: fetal sleep states, maternal medications eg, sedatives), or maternal smoking Kick counts ●Perception of least 10 fetal movements (FMs) over up to two hours when the mother is at rest and focused on counting ("count to 10" method) . Patients are instructed to contact within 12 hours for further evaluation if they perceive a significant and persistent reduction in fetal movement and never to wait longer than two hours if there is absent. DIFFERENTIAL DIAGNOSIS Transient decreases in fetal activity: -

Nonstress Test and Contraction Stress Test - Uptodate
2019/3/14 Nonstress test and contraction stress test - UpToDate Official reprint from UpToDate® www.uptodate.com ©2019 UpToDate, Inc. and/or its affiliates. All Rights Reserved. Nonstress test and contraction stress test Author: David A Miller, MD Section Editor: Charles J Lockwood, MD, MHCM Deputy Editor: Vanessa A Barss, MD, FACOG All topics are updated as new evidence becomes available and our peer review process is complete. Literature review current through: Feb 2019. | This topic last updated: Jan 16, 2018. INTRODUCTION Fetal health is evaluated, in part, by assessment of fetal heart rate (FHR) patterns. The primary goal is to identify fetuses at risk of intrauterine death or neonatal complications and intervene (often by delivery) to prevent these adverse outcomes, if possible. The nonstress test (NST) and the contraction stress test (CST) are used for antepartum FHR testing. An abnormal test (nonreactive NST, positive CST) is sometimes associated with adverse fetal or neonatal outcomes, while a normal test (reactive NST, negative CST) is usually associated with a neurologically intact and adequately oxygenated fetus. When interpreting these tests, the clinician needs to account for gestational age, prior results of fetal assessment, maternal conditions (including medications), and fetal condition (eg, growth restriction, anemia, arrhythmia). The NST and CST will be reviewed here. Intrapartum fetal evaluation and additional techniques for assessing fetal health are discussed separately. ● (See "Intrapartum fetal heart rate assessment".) ● (See "The fetal biophysical profile".) ● (See "Decreased fetal movement: Diagnosis, evaluation, and management".) ● (See "Doppler ultrasound of the umbilical artery for fetal surveillance".) PHYSIOLOGIC BASIS OF FETAL HEART RATE CHANGES Physiologic development of the fetal heart occurs across gestation and affects fetal heart rate (FHR) patterns. -
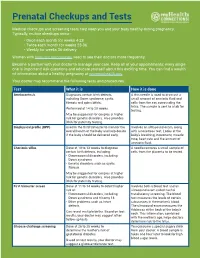
Prenatal Checkups and Tests
Prenatal Checkups and Tests Medical checkups and screening tests help keep you and your baby healthy during pregnancy. Typically, routine checkups occur: • Once each month for weeks 4-28 • Twice each month for weeks 28-36 • Weekly for weeks 36-delivery Women with high-risk pregnancies need to see their doctors more frequently. Become a partner with your doctor to manage your care. Keep all of your appointments: every single one is important! Ask questions and educate yourself about this exciting time. You can find a wealth of information about a healthy pregnancy at womenshealth.gov. Your doctor may recommend the following tests and procedures: Test What it is How it is done Amniocentesis Diagnoses certain birth defects, A thin needle is used to draw out a including Down syndrome, cystic small amount of amniotic fluid and fibrosis and spina bifida. cells from the sac surrounding the fetus. The sample is sent to a lab for Performed at 14 to 20 weeks. testing. May be suggested for couples at higher risk for genetic disorders. Also provides DNA for paternity testing. Biophysical profile (BPP) Used in the third trimester to monitor the Involves an ultrasound exam, along overall health of the baby and help decide with a nonstress test. Looks at the if the baby should be delivered early. baby’s breathing, movement, muscle tone, heart rate and the amount of amniotic fluid. Chorionic villus Done at 10 to 13 weeks to diagnose A needle removes a small sample of certain birth defects, including: cells from the placenta to be tested. -

The Predictive Value of Fetal Acoustic Stimulation
Original Article nnnnnnnnnnnnnn The Predictive Value of Fetal Acoustic Stimulation Ferit Sarac¸og˘lu, MD Objective clinical evaluation of fetal health is a primary goal of ob- Kemal Go¨l,MD stetric care. Because obstetricians recognize the association between I˙zzet S¸ahin, MD the presence of fetal heart rate (FHR) accelerations in response to fetal Bektas¸Tu¨rkkanı, MD movement and fetal well-being, FHR monitoring has been widely Cihan O¨ ztopc¸u, MD used for antenatal fetal surveillance. A major problem with antepartum FHR testing is the difficulty in separating healthy fetuses who can have prolonged periods of rest, OBJECTIVE: from sick fetuses who are not moving because of hypoxemia and/or To compare the predictive abilities, test duration times, and incidence of asphyxia. In the nonstress test (NST) there is a high incidence of nonreactive results in the acoustic stimulation test (AST) and the non- false-positive results. A healthy third trimester fetus is in a state of stress test (NST). quiet sleep approximately 30% to 40% of the time.1,2 Many unsuccess- ful attempts have been made to increase FHR reactivity and to de- METHOD: crease the length of NST. These include providing an external light,3 Four-hundred randomly selected patients, delivering within 7 days of a manipulating the fetus,4,5 and the maternal ingestion of glucose.6 preceding test, were divided into two groups (group I: NST; group II: Finally, several different fetal acoustic stimulation tests combined AST). In the AST group, fetal heart rate tracings were recorded for the with NST have been introduced in clinical practice.7 first 5 minutes as a baseline recording. -

And Its Corelation with Maternal and Fetal Outcome 1Navneet Kaur, 2Promila Jindal
JSAFOG Study of ‘Non Stress Test at Admission’ and its Corelation10.5005/jp-journals-10006-1581 with Maternal and Fetal Outcome ORIGINAL ARTICLE Study of ‘Nonstress Test at Admission’ and its Corelation with Maternal and Fetal Outcome 1Navneet Kaur, 2Promila Jindal ABSTRACT Outcome. J South Asian Feder Obst Gynae 2018;10(3):161- 166. Introduction: Non stress test (NST) is the most widely used test for assessment of fetal health and reflects oxygenation of Source of support: Nil brain. NST is usually recommended after 30 to 32 weeks of the Conflict of interest: None pregnancy. The false negative rate of NST (reactive NST in a fetus who actually is in distress) is 3.2/1000 which is very low Date of received: 09/24/2016 and thus NST is considered as a good predictor of fetal health. Date of acceptance: 11/06/2016 Objectives: To evaluate the “NST at admission” in all the Date of publication: December 2018 admitted women > 32 weeks of gestation and to correlate it with type of labour and mode of delivery and maternal and neonatal outcome. INTRODUCTION In modern obstetrics antenatal fetal surveillance is becom- Materials and methods: This prospective study was conducted on all the pregnant women only at > 32 weeks of gestation ing increasingly popular field for timely intervention admitted to Dayanand Medical College and Hospital (DMCH), for good fetal outcome. There are various modalities for Ludhiana from 1/1/2011 to 31/12/2011. Non stress test was done antenatal fetal surveillance but NS) has withstood the in all women using TOCODYNAMOMETER for 20 minutes and test of time since its introduction. -

FAQ069 -- What to Expect After Your Due Date
AQ The American College of Obstetricians and Gynecologists FREQUENTLY ASKED QUESTIONS FAQ069 PREGNANCY What to Expect After Your Due Date • What is the due date and what does it mean? • What is postterm pregnancy? f • What are the risks associated with postterm pregnancy? • What tests can be performed in cases of postterm pregnancy? • What is electronic fetal monitoring? • What is labor induction? • How is labor induced? • Glossary What is the due date and what does it mean? Your due date is used as a guide for checking your pregnancy’s progress and the baby’s growth and age. Health care providers often use more than one method to set the due date. Ultrasound performed between 18 weeks and 20 weeks of pregnancy often is used to help confirm the age of a fetus. What is postterm pregnancy? A postterm pregnancy is one that lasts 42 weeks or longer. Women who are having a baby for the first time or who have had postterm pregnancies before may give birth later than expected. However, the most common cause of postterm pregnancy is an error in calculating the due date. When a postterm pregnancy truly exists, the cause usually is unknown. What are the risks associated with postterm pregnancy? After 42 weeks, the placenta may not work as well as it did earlier in pregnancy. Also, as the baby grows, the amount of amniotic fluid may begin to decrease. Less fluid may cause the umbilical cord to become pinched as the baby moves or as the uterus contracts. If pregnancy goes past 42 weeks, a baby has an increased risk of certain problems, such as dysmaturity syndrome, macrosomia, or meconium aspiration. -

Appendix B Glossary of Terms, Diagnoses and Procedures
Appendix B Glossary of Terms, Diagnoses and Procedures This appendix contains basic information to assist non-medical members of the case review team to understand common terms, diagnoses and procedures that they might encounter in review of individual cases. (It may also be of use to the community action team members.) Local programs should feel free to add or delete items, as needed. Please do not feel that these terms need to be memorized. Use this document as a dictionary and refer to it as needed. Experience tells us that after a year or so of reviewing cases, all team members will natu- rally come to an understanding of these terms, as well as others, without making any special effort. Definitions in Appendix B are adopted from: American College of Obstetricians and Gynecologists. Your pregnancy and birth. 4th Ed. Washington, DC: ACOG; 2005 pp 353–361. Amniocentesis: A procedure in which a Bacterial Vaginosis: A type of vaginal in- small amount of amniotic fluid and cells are fection caused by the overgrowth of a num- taken from the sac surrounding the fetus ber of organisms that are normally found in and tested. the vagina. Amniotic Fluid: Water in the sac surrounding Bilirubin: A reddish-yellow pigment that the fetus in the mother’s uterus. occurs especially in bile and blood and may cause jaundice. Analgesics: A type of drug that relieves pain without loss of muscle function. Biophysical Profile: An assessment by ultra- sound of fetal breathing, fetal body move- Anemia: Abnormally low levels of blood or ments, fetal muscle tone and the amount of red blood cells in the bloodstream. -

The Effect of Different Maternal Positions on Reactivity of the Nonstress Test, Maternal Blood Pressure and Heart Rate
İzmir Dr. Behçet Uz Çocuk Hast. Dergisi 2018;8(2):101-108 Araştırma doi:10.5222/buchd.2018.101 The effect of different maternal positions on reactivity of the nonstress test, maternal blood pressure and heart rate Farklı maternal pozisyonların nonstres test reaktivitesi, maternal kan basıncı ve kalp atım hızına etkisi Didem KIRATLI1, Tülay YAVAN2, Kazım Emre KARAŞAHİN3, Müfit Cemal YENEN4 1Dr. Behçet Uz Çocuk Hastanesi, İzmir, Türkiye 2İzmir Ekonomi Üniversitesi, Sağlık Bilimleri Fakültesi, İzmir, Türkiye 3Sağlık Bilimleri Üniversitesi, Gülhane Eğitim ve Araştırma Hastanesi, Kadın Hastalıkları ve Doğum Kliniği, Ankara, Türkiye 4Girne Üniversitesi Dr. Suat Günsel Hastanesi, Kadın Hastalıkları ve Doğum Kliniği, Girne, KKTC ABSTRACT Objective: We aimed to investigate the effects of different maternal positions on reactivity of the Nonstress Test (NST), maternal blood pressure and heart rate. Methods: In this experimental study, 243 pregnants were randomized to the position groups (sitting, semi-Fowler’s, semi-Fowler’s left lateral) at 34-37 and 38-40 gestational weeks. The questionnaire form inquiring socio-demographic and obstetric characteristics and “NST Form” were used. For the statistical comparison of the continuous variables, one-way ANOVA, and independent groups t test were used. Results: Though not statistically significant difference existed, NST reactivity at sitting position was higher than the other positions, and it was higher in 38-40 gestational weeks relative to 34-37 gestational weeks (p>0.05). Results of NST reactivity were evaluated at 4 time periods. Time-to-reactivity in relation to position didn’t reveal a significant difference. Independent of the position, we found a significant difference in time to reactivity between 0-5 and 0-10 minutes, and between 0-10 and 0-15 minutes (p<0.05), but there was no signi- ficant difference in time to reactivity between 0-15 and 0-20 minutes (p>0.05). -
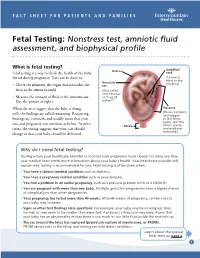
Fetal Testing: Nonstress Test, Amniotic Fluid Assessment, and Biophysical Profile
FACT SHEET FOR PATIENTS AND FAMILIES Fetal Testing: Nonstress test, amniotic fluid assessment, and biophysical profile What is fetal testing? Uterus Umbilical Fetal testing is a way to check the health of the baby cord (fetus) during pregnancy. Tests can be done to: (Connects fetus to the Amniotic placenta) • Check the placenta, the organ that nourishes the sac fetus in the uterus (womb). (Also called “membranes” • Measure the amount of fluid in the amniotic sac. or “bag of (See the picture at right.) waters”) When the tests suggest that the baby is doing Placenta (Passes nutrients reassuring well, the findings are called . Reassuring and oxygen findings are common, and usually mean that your to the fetus, helps clear the care and pregnancy can continue as before. At other Cervix fetus’s waste, times, the testing suggests that your care should and produces hormones) change or that your baby should be delivered. Why do I need fetal testing? Testing allows your healthcare provider to monitor your pregnancy more closely. It’s done any time your medical team needs more information about your baby’s health. Your healthcare provider will explain why testing is recommended for you. Fetal testing is often done when: • You have a chronic medical condition such as diabetes. • You have a pregnancy-related condition such as preeclampsia. • You had a problem in an earlier pregnancy, such as a previous preterm birth or a stillbirth. • You are pregnant with more than one baby. Multiple gestation pregnancies have a higher chance of complications than other pregnancies. • Your pregnancy has lasted more than 40 weeks.