Hypothesis on the Pathophysiology of Syringomyelia Based on Simulation of Cerebrospinal Fluid Dynamics H S Chang, H Nakagawa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production
The Journal of Neuroscience, March 25, 2015 • 35(12):4903–4916 • 4903 Development/Plasticity/Repair Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production Melody P. Lun,1,3 XMatthew B. Johnson,2 Kevin G. Broadbelt,1 Momoko Watanabe,4 Young-jin Kang,4 Kevin F. Chau,1 Mark W. Springel,1 Alexandra Malesz,1 Andre´ M.M. Sousa,5 XMihovil Pletikos,5 XTais Adelita,1,6 Monica L. Calicchio,1 Yong Zhang,7 Michael J. Holtzman,7 Hart G.W. Lidov,1 XNenad Sestan,5 Hanno Steen,1 XEdwin S. Monuki,4 and Maria K. Lehtinen1 1Department of Pathology, and 2Division of Genetics, Boston Children’s Hospital, Boston, Massachusetts 02115, 3Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston, Massachusetts 02118, 4Department of Pathology and Laboratory Medicine, University of California Irvine School of Medicine, Irvine, California 92697, 5Department of Neurobiology and Kavli Institute for Neuroscience, Yale School of Medicine, New Haven, Connecticut 06510, 6Department of Biochemistry, Federal University of Sa˜o Paulo, Sa˜o Paulo 04039, Brazil, and 7Pulmonary and Critical Care Medicine, Department of Medicine, Washington University, St Louis, Missouri 63110 A sheet of choroid plexus epithelial cells extends into each cerebral ventricle and secretes signaling factors into the CSF. To evaluate whether differences in the CSF proteome across ventricles arise, in part, from regional differences in choroid plexus gene expression, we defined the transcriptome of lateral ventricle (telencephalic) versus fourth ventricle (hindbrain) choroid plexus. We find that positional identitiesofmouse,macaque,andhumanchoroidplexiderivefromgeneexpressiondomainsthatparalleltheiraxialtissuesoforigin.We thenshowthatmolecularheterogeneitybetweentelencephalicandhindbrainchoroidplexicontributestoregion-specific,age-dependent protein secretion in vitro. -

Human Cerebrospinal Fluid Induces Neuronal Excitability Changes in Resected Human Neocortical and Hippocampal Brain Slices
bioRxiv preprint doi: https://doi.org/10.1101/730036; this version posted August 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Human cerebrospinal fluid induces neuronal excitability changes in resected human neocortical and hippocampal brain slices Jenny Wickham*†1, Andrea Corna†1,2,3, Niklas Schwarz4, Betül Uysal 2,4, Nikolas Layer4, Thomas V. Wuttke4,5, Henner Koch4, Günther Zeck1 1 Neurophysics, Natural and Medical Science Institute (NMI) at the University of Tübingen, Reutlingen, Germany 2 Graduate Training Centre of Neuroscience/International Max Planck Research School, Tübingen, Germany. 3 Institute for Ophthalmic Research, University of Tübingen, Tübingen, Germany 4 Department of Neurology and Epileptology, Hertie-Institute for Clinical Brain Research, University of Tübingen, Tübingen, Germany 5 Department of Neurosurgery, University of Tübingen, Tübingen, Germany * Communicating author: Jenny Wickham ([email protected]) and Günther Zeck ([email protected]) † shared first author, Shared last author Acknowledgments: This work was supported by the German Ministry of Science and Education (BMBF, FKZ 031L0059A) and by the German Research Foundation (KO-4877/2-1 and KO 4877/3- 1) Author contribution: JW and AC performed micro-electrode array recordings, NS, BU, NL, TW, HK and JW did tissue preparation and culturing, JW, AC, GZ did data analysis and writing. Proof reading and study design: all. Conflict of interest: None Keywords: Human tissue, human cerebrospinal fluid, Microelectrode array, CMOS-MEA, hippocampus, cortex, tissue slice 1 bioRxiv preprint doi: https://doi.org/10.1101/730036; this version posted August 8, 2019. -

Cerebrospinal Fluid in Critical Illness
Cerebrospinal Fluid in Critical Illness B. VENKATESH, P. SCOTT, M. ZIEGENFUSS Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To detail the physiology, pathophysiology and recent advances in diagnostic analysis of cerebrospinal fluid (CSF) in critical illness, and briefly review the pharmacokinetics and pharmaco- dynamics of drugs in the CSF when administered by the intravenous and intrathecal route. Data Sources: A review of articles published in peer reviewed journals from 1966 to 1999 and identified through a MEDLINE search on the cerebrospinal fluid. Summary of review: The examination of the CSF has become an integral part of the assessment of the critically ill neurological or neurosurgical patient. Its greatest value lies in the evaluation of meningitis. Recent publications describe the availability of new laboratory tests on the CSF in addition to the conventional cell count, protein sugar and microbiology studies. Whilst these additional tests have improved our understanding of the pathophysiology of the critically ill neurological/neurosurgical patient, they have a limited role in providing diagnostic or prognostic information. The literature pertaining to the use of these tests is reviewed together with a description of the alterations in CSF in critical illness. The pharmacokinetics and pharmacodynamics of drugs in the CSF, when administered by the intravenous and the intrathecal route, are also reviewed. Conclusions: The diagnostic utility of CSF investigation in critical illness is currently limited to the diagnosis of an infectious process. Studies that have demonstrated some usefulness of CSF analysis in predicting outcome in critical illness have not been able to show their superiority to conventional clinical examination. -

Hydrocephalus Patient Information Guide Experience Life Without Boundaries
Hydrocephalus Patient Information Guide Experience Life Without Boundaries Aesculap Neurosurgery 2 Table of Contents Foreword Page 4 About Us Page 5 What Is Hydrocephalus Page 6 Types Of Hydrocephalus Page 7 What You Should Know Page 8 Diagnosis Page 9 Treatment/Goal/Surgical Procedure Page 10 Complications Page 10 Prognosis/Helpful Sites Page 11 The Aesculap Shunts Page 12 Medical Definitions Page 13 3 Foreword The intention of this booklet is to provide information to patients, family members, caregivers and friends on the subject of hydrocephalus. The information provided is a general overview of the diagnosis and treatment of hydrocephalus and other conditions associated with hydrocephalus. About us History Aesculap AG (Germany) was founded in 1867 by Gottfried Jetter, a master craftsman trained in surgical instrument and cutlery techniques. Jetter’s original workshop, located in Tuttlingen, Germany, is the same location where Aesculap world headquarters resides today. The employee count has increased over the years (3,000+ employees), and the number of instrument patterns has grown from a select few to over 17,000; however, the German standard for quality and pattern consistency remains the same. Prior to 1977, many of the instruments sold in America by competing companies were sourced from Aesculap in Tuttlingen. In response to the United States customer demand for Aesculap quality surgical instruments, Aesculap, Inc. (U.S.) was established in 1977. Headquartered in Center Valley, Pennsylvania, with over one hundred direct nationwide sales representatives, Aesculap, Inc. currently supports the marketing, sales and distribution of Aesculap surgical instrumentation in the U.S. In order to support the company’s continued growth and provide the service and quality which customers have come to expect, Aesculap relocated the corporate offices from San Francisco to Center Valley, Pennsylvania. -

MENINGES and CEREBROSPINAL FLUID' by LEWIS H
MENINGES AND CEREBROSPINAL FLUID' By LEWIS H. WEED Department of Anatomy, John Hopkins University THE divorce of structure from function is particularly difficult in any ana- tomical study: it was only 85 years ago that the two subjects of morphology and physiology were considered to justify separate departments as academic disciplines. But with this cleavage which fortunately has not at any time been a rigid one, only certain investigations could go forward without loss of in- spiration and interpretation when studied apart from the sister science; other researches were enormously hampered and could be attacked only with due regard to structure and function. So it is without apologies that I begin the presentation of the problem of the coverings of the central nervous system -coverings which encompass a characteristic body fluid. Here then is a problem of membranes serving to contain a clear, limpid liquid as a sac might hold it. Immediately many questions of biological significance are at hand: how does it happen that these structures retain fluid; where does the fluid come from; where does it go; is the fluid constantly produced or is it an inert, non-circu- lating medium; is the fluid under pressure above that of the atmosphere; does it move about with changes in the animal body?-but the list of problems springing into one's mind grows too long. Knowledge regarding these many questions has progressed since the first accounts of hydrocephalus were given by writers in the Hippocratic corpus, since discovery of the normal ventricular fluid in Galen's time, since its meningeal existence was first uncovered by Valsalva (1911) and advanced by Cotugno (1779), since the first adequate description by Magendie (1825) 100 years ago. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Mathematical Modelling and Analysis of Cerebrospinal Mechanics: an Investigation Into the Pathogenesis of Syringomyelia
University of Warwick institutional repository: http://go.warwick.ac.uk/wrap A Thesis Submitted for the Degree of PhD at the University of Warwick http://go.warwick.ac.uk/wrap/2751 This thesis is made available online and is protected by original copyright. Please scroll down to view the document itself. Please refer to the repository record for this item for information to help you to cite it. Our policy information is available from the repository home page. Mathematical Modelling and Analysis of Cerebrospinal Mechanics: An Investigation Into the Pathogenesis of Syringomyelia Novak Samuel Jon Elliott BSc(Hons) BE(Hons) MBiomedE This report is submitted as partial fulfilment of the requirements for the PhD Programme of the School of Engineering University of Warwick August 2009 AUTHOR: Novak Samuel Jon Elliott DEGREE: PhD TITLE: Mathematical Modelling and Analysis of Cerebrospinal Me- chanics: An Investigation Into the Pathogenesis of Syringomyelia DATE OF DEPOSIT: ............................. I agree that this thesis shall be available in accordance with the regulations gov- erning the University of Warwick theses. I agree that the summary of this thesis may be submitted for publication. I agree that the thesis may be photocopied (single copies for study purposes only). Theses with no restriction on photocopying will also be made available to the British Library for microfilming. The British Library may supply copies to individuals or li- braries. subject to a statement from them that the copy is supplied for non-publishing purposes. All copies supplied by the British Library will carry the following statement: \Attention is drawn to the fact that the copyright of this thesis rests with its author. -

Cerebrospinal Fluid Collection Fact Sheet Memory & Aging Project (MAP), Washington University
Cerebrospinal Fluid Collection Fact Sheet Memory & Aging Project (MAP), Washington University What is cerebrospinal fluid? Cerebrospinal fluid (CSF) is made in the brain to protect the brain and spinal cord. CSF contains proteins and other chemicals that are important for brain health. The type and amount of these proteins and chemicals may indicate a disease process, such as Alzheimer disease (AD) or potential risk for AD. The analysis of CSF provides a unique “window” into the understanding of how AD develops and progresses. Findings about AD in living people through the study of CSF mean that the illness may be diagnosed during life, without waiting to confirm the diagnosis at autopsy after death. How is CSF collected? Base of the Experienced clinicians collect CSF by a lumbar puncture (LP), Spinal Cord also called a spinal tap. Research volunteers generally have the LP while sitting up and fully awake. The back is cleaned and a numbing medication is injected into the skin. When Cerebrospinal Fluid Small Needle the skin is numb, a thin needle is inserted into the back at the level of the hip bones. There is no spinal cord at that level, and LP involves inserting a small needle between the vertebrae below the there is no risk for paralysis. The needle is placed between base of spinal cord. A small amount (not through) the bones of the spine until the spinal fluid is of fluid is collected. There is no risk for paralysis. reached. For testing, approximately 2-3 tablespoons of fluid are collected in sterile tubes. The brain makes CSF constantly, and at any one time over 37 tablespoons are available. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
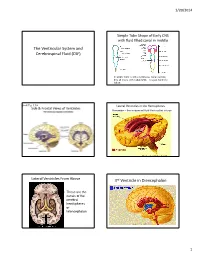
The Ventricular System and Cerebrospinal Fluid (CSF) 3Rd Ventricle in Diencephalon
1/20/2014 Simple Tube Shape of Early CNS with fluid filled canal in middle The Ventricular System and Cerebrospinal Fluid (CSF) In adults there is still a continuous canal running thru all levels of the adult CNS – it is just harder to follow. Book Fig. 1.19 Lateral Ventricles in the Hemispheres Side & Frontal Views of Ventricles Remember – these represent fluid filled cavities in brain “Wishbone” shape means there is ventricle within each of the lobes. Lateral Ventricles From Above 3rd Ventricle in Diencephalon • These are the canals of the cerebral hemispheres or telencephalon 1 1/20/2014 Interventricular foramen links lateral ventricles to 3rd You can see the 2 dark lateral ventricles as well as the vertical midline 3 rd ventricle Sagittal Section Thru 3 rd Vent. (#2 in figure) • Connects to skinny cerebral aqueduct, the canal of the midbrain (just under the #10) Choroid plexus located in ventricles continuously 4th Ventricle in the Hindbrain produces CSF, replacing the ~125-150 ml several times/day Normal pressure 3 holes in the roof of CSF: of the 4 th venticle 100-150 mmH2O allow most CSF to lying down leave ventricles 200-300 mmH2O and enter the sitting up subarachnoid space around brain 2 1/20/2014 Circulation of CSF in • Why is CSF pressure measured in mm H2O? • Larger pressure differences (like BP) are ventricles measured by the movement of a column of always heavier liquid (Mercury or Hg) moves • Smaller pressure differences are measured by the movement of a column of lighter liquid (H2O) posteriorly, – CSF moves that column ~150 mm but would toward only move Hg about 2 mm. -

CEREBROSPINAL FLUID (CSF) LEAK Introduction: Cerebrospinal
© 2013 UT Southwestern Medical Center. Mktg 305_3 © 2013 UT Southwestern Medical Center. Comprehensive Skull Base Program • 214-645-3400 • utswmedicine.org/skullbase CEREBROSPINAL FLUID (CSF) LEAK Introduction: Cerebrospinal fluid fistula, commonly known as CSF leak, occurs when there is an abnormal communication between the CSF containing space around the brain (subarachnoid space) and either the sinuses or the ear. In general, the brain is a sterile compartment and is separated from the sinuses and the ear by a bony separation called the skull base. Cerebrospinal fluid surrounds the brain and cushions it. A thick, leathery tissue called dura mater (or just “dura,” for short) lines the inside of the skull. Dura provides a waterproof seal that prevents spinal fluid from escaping. CSF leaks occur when there is a breakdown of this barrier. CSF leaks may be recognized as clear drainage coming from the nose (CSF rhinorrhea) or the ear (CSF otorrhea). CSF leaks most commonly occur from trauma or surgery; however, they can also occur spontaneously without an identifiable cause. Untreated CSF leaks can represent a potentially life threatening situation leading to meningitis, brain infection, stroke and death. Thus, timely diagnosis and repair is warranted to minimize this risk. In the past, repair often required brain surgery, but now many cases of CSF rhinorrhea can be repaired by working through the nose and sinuses with endoscopic instruments (i.e., no external incisions). CSF Rhinorrhea: CSF rhinorrhea results due to a breakdown of the barrier separating the spinal fluid space from the nose and sinuses. The usual symptom is watery, nasal drainage coming from either the left or right side of the nose. -

Brachycephaly and Cerebrospinal Fluid Disorders
life Review The Need for Head Space: Brachycephaly and Cerebrospinal Fluid Disorders Clare Rusbridge 1,2,* and Penny Knowler 1 1 Faculty of Health & Medical Sciences, School of Veterinary Medicine, University of Surrey, Guildford GU2 7AL, UK; [email protected] 2 Fitzpatrick Referrals, Godalming GU7 2QQ, UK * Correspondence: [email protected] Abstract: Brachycephalic dogs remain popular, despite the knowledge that this head conformation is associated with health problems, including airway compromise, ocular disorders, neurological dis- ease, and other co-morbidities. There is increasing evidence that brachycephaly disrupts cerebrospinal fluid movement and absorption, predisposing ventriculomegaly, hydrocephalus, quadrigeminal cistern expansion, Chiari-like malformation, and syringomyelia. In this review, we focus on cere- brospinal fluid physiology and how this is impacted by brachycephaly, airorhynchy, and associated craniosynostosis. Keywords: ventriculomegaly; hydrocephalus; Chiari malformation; syringomyelia; canine; cran- iosynostosis; supracollicular fluid collection; quadrigeminal cistern; lateral aperture; sleep disordered breathing; brachycephalic obstructive airway disease 1. Introduction Brachycephalic dogs and cats have proved to be increasingly popular since they Citation: Rusbridge, C.; Knowler, P. were introduced to Europe in Victorian times. This rising popularity defies a high preva- The Need for Head Space: lence of conformation-related morbidity, including breathing, ocular, and neurological Brachycephaly and Cerebrospinal disorders [1,2]. Brachycephaly in domestic pets is a consequence of selecting for juvenile Fluid Disorders. Life 2021, 11, 139. characteristics of a flattened face and a rounded head [3]. Calvarial doming associated https://doi.org/10.3390/life11020139 with wide zygomatic arches and a wide, flattened, or convex palate is compensation for premature closure of skull-base sutures, including the basispheno-presphenoid syn- Academic Editor: Edgar Lehr chondrosis and spheno-occipital synchondrosis [4,5].