EMS Scope of Practice Guidelines for Drugs
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

First Responder (2013)
THE NATIONAL ACADEMIES PRESS This PDF is available at http://nap.edu/22451 SHARE The Legal Definitions of First Responder (2013) DETAILS 30 pages | 8.5 x 11 | PAPERBACK ISBN 978-0-309-28369-4 | DOI 10.17226/22451 CONTRIBUTORS GET THIS BOOK Bricker, Lew R. C.; Petermann, Tanya N.; Hines, Margaret; and Sands, Jocelyn FIND RELATED TITLES SUGGESTED CITATION National Academies of Sciences, Engineering, and Medicine 2013. The Legal Definitions of First Responder . Washington, DC: The National Academies Press. https://doi.org/10.17226/22451. Visit the National Academies Press at NAP.edu and login or register to get: – Access to free PDF downloads of thousands of scientific reports – 10% off the price of print titles – Email or social media notifications of new titles related to your interests – Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. (Request Permission) Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Copyright © National Academy of Sciences. All rights reserved. The Legal Definitions of “First Responder” November 2013 NATIONAL COOPERATIVE HIGHWAY RESEARCH PROGRAM Responsible Senior Program Officer: Stephan A. Parker Research Results Digest 385 THE LEGAL DEFINITIONS OF “FIRST RESPONDER” This digest presents the results of NCHRP Project 20-59(41), “Legal Definition of ‘First Responder’.” The research was conducted by Lew R. C. Bricker, Esquire, and Tanya N. Petermann, Esquire, of Smith Amundsen, Chicago, IL; Margaret Hines, Esquire; and Jocelyn Sands, J. D. James B. McDaniel was the Principal Investigator. INTRODUCTION Congress and in some congressional bills that were not enacted into law. -

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -
![Doxapram Shortens Recovery Following Sevoflurane Anesthesia [Le Doxapram Hâte La Récupération Après Une Anesthésie Au Sévoflurane]](https://docslib.b-cdn.net/cover/1653/doxapram-shortens-recovery-following-sevoflurane-anesthesia-le-doxapram-h%C3%A2te-la-r%C3%A9cup%C3%A9ration-apr%C3%A8s-une-anesth%C3%A9sie-au-s%C3%A9voflurane-391653.webp)
Doxapram Shortens Recovery Following Sevoflurane Anesthesia [Le Doxapram Hâte La Récupération Après Une Anesthésie Au Sévoflurane]
456 CANADIAN JOURNALGENERAL OF ANESTHESIA ANESTHESIA Doxapram shortens recovery following sevoflurane anesthesia [Le doxapram hâte la récupération après une anesthésie au sévoflurane] Chi-Chen Wu MD,* Martin S. Mok MD,* Jui-Yuan Chen MD,† Gong-Jhe Wu MD,† Yeong-Ray Wen MD,† Chao-Shun Lin MD* Purpose: A randomized, double blind controlled trial was possibilité de serrer la main sur demande, le temps d’extubation et undertaken to investigate the effect of doxapram on recovery le score de récupération d’Aldrete. Les valeurs de l’index bispectral, times and bispectral index following sevoflurane anesthesia. la tension artérielle systolique et la fréquence cardiaque ont été Methods: Upon completion of surgery under sevoflurane anes- enregistrées avant l’anesthésie, pendant l’opération et à chaque thesia, 60 adult patients were randomly allocated to receive minute pendant 15 min après l’administration du médicament. either doxapram hydrochloride 1 mg·kg–1 iv or saline placebo. Résultats : Le temps écoulé avant l’ouverture des yeux a été plus Clinical recovery from anesthesia was assessed by time to eye court avec le doxapram qu’avec le placebo (6,9 ± 2,2 min vs 9,9 opening on verbal command, hand squeezing on command, ± 3,1 min, P < 0,05). Les scores moyens de l’index bispectral ont time to extubation, and the Aldrete recovery score. Bispectral été aussi plus élevés avec le doxapram sept à huit minutes après index values, systolic blood pressure, and heart rate were l’administration du médicament expérimental (P < 0,05). Un recorded at baseline (before anesthesia), during surgery, and retour à la conscience plus rapide a été associé à une plus grande every minute for 15 min after administration of the study drug. -

Abcd (Ipratropium Bromide and Albuterol Sulfate) Inhalation Aerosol
ATTENTION PHARMACIST: Detach “Patient’s Instructions for Use” from package insert and dispense with the product. Combivent® abcd (ipratropium bromide and albuterol sulfate) Inhalation Aerosol Bronchodilator Aerosol For Oral Inhalation Only Rx only Prescribing Information DESCRIPTION COMBIVENT Inhalation Aerosol is a combination of ipratropium bromide (as the monohydrate) and albuterol sulfate. Ipratropium bromide is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo[3.2.1] octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl 8-(1-methylethyl)-, bromide monohydrate, (3-endo, 8-syn)-: a synthetic quaternary ammonium compound chemically related to atropine. Ipratropium bromide is a white to off-white crystalline substance, freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in lipophilic solvents such as ether, chloroform, and fluorocarbons. The structural formula is: + N OH Br . H O H 2 O O C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4 Albuterol sulfate, chemically known as (1,3-benzenedimethanol, α'-[[(1,1dimethylethyl) amino] methyl]-4-hydroxy, sulfate (2:1)(salt), (±)- is a relatively selective beta2-adrenergic bronchodilator. Albuterol is the official generic name in the United States. The World Health Organization recommended name for the drug is salbutamol. Albuterol sulfate is a white to off-white crystalline powder, freely soluble in water and slightly soluble in alcohol, chloroform, and ether. The structural formula is: Reference ID: 2927161 OH NH * . H2SO4 OH OH 2 (C13H21NO3)2•H2SO4 albuterol sulfate Mol. Wt. 576.7 Combivent® (ipratropium bromide and albuterol sulfate) Inhalation Aerosol contains a microcrystalline suspension of ipratropium bromide and albuterol sulfate in a pressurized metered-dose aerosol unit for oral inhalation administration. -

Emergency Medical System 2021 Patient Treatment Protocols
2021 Patient Treatment Protocols Effective January 1, 2021 CONTENTS Table of Contents Preface Section ...........................................................................................................00.000 EMS Provider Scope of Practice and Nomenclature .....................................................00.010 Death in the Field ........................................................................................................00.020 Dying and Death, POLST, Do Not Attempt Resuscitation Orders ..............................00.030 Medical Control for Drugs and Procedures ..................................................................00.040 Treatment ......................................................................................................... Section 10.000 Abdominal Pain ...........................................................................................................10.010 Altered Mental Status and Coma ..................................................................................10.020 Anaphylaxis and Allergic Reaction ................................................................................10.030 Burns ...........................................................................................................................10.040 Cardiac Arrest ..............................................................................................................10.050 Emergency Medical Responder/EMT Paramedic/EMT-Intermediate Quick Reference to Pediatric Drugs Cardiac Dysrhythmias ..................................................................................................10.060 -

MASS CASUALTY TRAUMA TRIAGE PARADIGMS and PITFALLS July 2019
1 Mass Casualty Trauma Triage - Paradigms and Pitfalls EXECUTIVE SUMMARY Emergency medical services (EMS) providers arrive on the scene of a mass casualty incident (MCI) and implement triage, moving green patients to a single area and grouping red and yellow patients using triage tape or tags. Patients are then transported to local hospitals according to their priority group. Tagged patients arrive at the hospital and are assessed and treated according to their priority. Though this triage process may not exactly describe your agency’s system, this traditional approach to MCIs is the model that has been used to train American EMS As a nation, we’ve got a lot providers for decades. Unfortunately—especially in of trailers with backboards mass violence incidents involving patients with time- and colored tape out there critical injuries and ongoing threats to responders and patients—this model may not be feasible and may result and that’s not what the focus in mis-triage and avoidable, outcome-altering delays of mass casualty response is in care. Further, many hospitals have not trained or about anymore. exercised triage or re-triage of exceedingly large numbers of patients, nor practiced a formalized secondary triage Dr. Edward Racht process that prioritizes patients for operative intervention American Medical Response or transfer to other facilities. The focus of this paper is to alert EMS medical directors and EMS systems planners and hospital emergency planners to key differences between “conventional” MCIs and mass violence events when: • the scene is dynamic, • the number of patients far exceeds usual resources; and • usual triage and treatment paradigms may fail. -

Revista Brasileira De Anestesiologia
Rev BrasBras Anestesiol. Anestesiol. 2013;63(1):163–164 2013;63(1):163-166 REVISTA BRASILEIRA DE ANESTESIOLOGIA Offi cial Publication of the Brazilian Society of Anesthesiology www.sba.com.br/rba/index.asp LETTER TO THE EDITOR Precipitation in Gallipoli: Sugammadex / Amiodarone & Sugammadex / Dobutamine & Sugammadex / Protamine Dear Editor, furosemide (10 mg.mL-1), gentamicin (40 mg.mL-1), glyceryl Sugammadex is a modifi ed gamma cyclodextrin 1-3. Cyclodextrins trinitrate (5 mg.mL-1), heparin (1,000 IU.mL-1), hydrocor- are water soluble cyclic oligosaccharides with a lipophilic tisone (250 mg.mL-1), crystallized insulin (100 IU.mL-1), core. Sugammadex has quickly found a place in clinical use Calcium (Calcium Gluconate Monohydrate 225 mg.10 mL-1 for selective antagonism of neuromuscular blockade with ro- + Calcium levulinate dihitrate 572 mg.10 mL-1), ketamine curonium 1-3. Sugammadex quickly encapsulates steroidal neu- (50 mg.mL-1), levobupivacaine (7.5 mg.mL-1), magnesium romuscular blockers, increasing the amount of encapsulated sulphate (1.2 mEq.mL-1), metamizol sodium (0.5 g.mL-1), steroidal neuromuscular blockers in plasma and separating the methylergobasin maleate (0.2 mg.mL-1), metoclopramide blockers from the nicotinic acetylcholine receptors 1-3. (5 mg.mL-1), metoprolol (1 mg.mL-1), morphine (0.01 g.mL-1), Apart from its use with steroidal neuromuscular block- midazolam (5 mg.mL-1), n-acetylcysteine (100 mg.mL-1), ers, it is known that sugammadex interacts with over 40 naloxone (0.4 mg.mL-1), neostigmine (0.5 mg.mL-1), nitroprus- lipophilic, steroidal and non-steroidal drugs. -

Pharmacology of Ophthalmic Agents
Ophthalmic Pharmacology Richard Alan Lewis M.D., M.S., PHARMACOLOGY FOPS PHARMACOKINETICS OF Professor, Departments of Ophthalmology, • The study of the absorption, OPHTHALMIC Medicine, Pediatrics, and Molecular distribution, metabolism, AGENTS and Human Genetics and excretion of a drug or and the National School of Tropical agent Introduction and Review Medicine Houston, Texas PHARMACOKINETICS Factors Affecting Drug Penetration Factors Affecting Drug Penetration into Ocular Tissues • A drug can be delivered to ocular tissue: into Ocular Tissues – Locally: • Drug concentration and solubility: The higher the concentration the better the penetration, • Surfactants: The preservatives in ocular • Eye drop but limited by reflex tearing. preparations alter cell membrane in the cornea • Ointment and increase drug permeability, e.g., • Viscosity: Addition of methylcellulose and benzalkonium and thiomersal • Periocular injection polyvinyl alcohol increases drug penetration by • pH: The normal tear pH is 7.4; if the drug pH is • Intraocular injection increasing the contact time with the cornea and altering corneal epithelium. much different, it will cause reflex tearing. – Systemically: • Lipid solubility: Because of the lipid rich • Drug tonicity: When an alkaloid drug is put in • Orally environment of the epithelial cell membranes, relatively alkaloid medium, the proportion of the uncharged form will increase, thus more • IM the higher lipid solubility, the more the penetration. • IV penetration. FLUORESCEIN FLUORESCEIN Chemistry Dosage ● C20H1205, brown crystal ● Adults: 500-750 mg IV ● M.W. 322.3 e.g., 3 cc 25% solution ● Peak absorption 485-500 nm. 5 cc 10% solution ● Peak emission 520-530 nm. ● Children: 1.5-2.5 mg/kg IV Richard Alan Lewis, M.D., M.S. -

“Inactive” Ingredients in Pharmaceutical Products: Update (Subject Review)
AMERICAN ACADEMY OF PEDIATRICS Committee on Drugs “Inactive” Ingredients in Pharmaceutical Products: Update (Subject Review) ABSTRACT. Because of an increasing number of re- bronchospasm from antiasthmatic drugs, aspartame- ports of adverse reactions associated with pharmaceutical induced headache and seizures, saccharin-induced excipients, in 1985 the Committee on Drugs issued a cross-sensitivity reactions in children with sulfon- position statement1 recommending that the Food and amide allergy, benzyl alcohol toxicity in neonates Drug Administration mandate labeling of over-the- receiving high-dose continuous infusion with pre- counter and prescription formulations to include a qual- served medications, dye-related cross-reactions in itative list of inactive ingredients. However, labeling of inactive ingredients remains voluntary. Adverse reac- children with aspirin intolerance, lactose-induced di- tions continue to be reported, although some are no arrhea, and propylene glycol-induced hyperosmola- longer considered clinically significant, and other new lity and lactic acidosis. Although many other excipi- reactions have emerged. The original statement, there- ents have been implicated in causing adverse fore, has been updated and its information expanded. reactions, these are the most significant in the pedi- atric population. ABBREVIATIONS. FDA, Food and Drug Administration; MDIs, metered-dose inhalers ANTIASTHMATIC MEDICATIONS It is readily appreciated that some percentage of asthmatic children will develop a “paradoxical” Pharmaceutical products often contain agents that bronchospasm after they inhale their medication. Be- have a variety of purposes, including improvement cause many of these reactions were attributed to of the appearance, bioavailability, stability, and pal- sulfite, which had been highly publicized as a caus- atability of the product. Excipients (substances ative agent, it was often first suspected. -
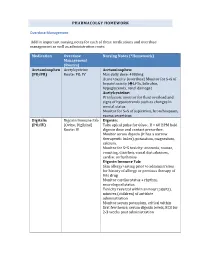
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Doxapram Elisa Kit Instructions Product #106219 & 106216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology DOXAPRAM ELISA KIT INSTRUCTIONS PRODUCT #106219 & 106216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Doxapram and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Doxapram ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug-enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution.