Municipal Solid Waste Incineration (MSWI) Ashes for Greener Concrete Master’S Project for the Master Program of Structural
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Opportunities for Geologic Carbon Sequestration in Washington State
Opportunities for Geologic Carbon Sequestration in Washington State Contributors: Jacob R. Childers, Ryan W. Daniels, Leo F. MacLeod, Jonathan D. Rowe, and Chrisopher R. Walker Faculty Advisor: Juliet G. Crider Department of Earth and Space Sciences University of Washington, Seattle May 2020 ESS Special Topics Task Force Report 001 Preface and Acknowledgment This report is the product of reading, conversation, writing and revision by a group of undergraduate student authors during 10 weeks of March, April and May 2020. Our intent is to review the basic processes and state of current scientific understanding of geologic carbon sequestration relevant to Washington State. We would like to thank Dr. Thomas L. Doe (Golder Associates) for reading the final report and asking us challenging questions. i I. Introduction Anthropogenic emissions of greenhouse gasses like CO2 are raising global temperatures at an unprecedented rate. According to the most recent United Nations Intergovernmental Panel on Climate Change report, global emissions have caused ~1°C global warming above pre-industrial levels, with impacts such as rising sea level and changing weather patterns (IPCC 2018). The IPCC states that the current rate of emissions will lead to global warming of 1.5°C sometime between 2030 to 2052, and that temperature will continue to rise above that if emissions are not halted. They state that impacts of global warming, such as drought or extreme precipitation, will increase with a 1.5°C temperature increase. However, these effects will be less than the impacts of global temperature increase of beyond 2°C. The IPCC also projects that global sea level rise will be 0.1 m lower at 1.5°C warming than at 2°C warming, exposing 10 million fewer people to risks related to sea level rise. -

BOOK of ABSTRACTS SCK•CEN-BA-53 13/Dja/P-23
BOOK OF ABSTRACTS SCK•CEN-BA-53 13/Dja/P-23 3rd International Workshop Mechanisms and modelling of waste/cement interactions Ghent, 06-08 May 2013 SCK•CEN Boeretang 200 BE-2400 MOL Belgium http://www.sckcen.be BOOK OF ABSTRACTS SCK•CEN-BA-53 3rd International Workshop Mechanisms and modelling of waste/cement interactions Ghent, 06-08 May 2013 SCK•CEN, Boeretang 200, BE-2400 MOL, Belgium Contact: Diederik Jacques Tel: +32 14 33 32 09 E-mail: [email protected] Organising committee Workshop convenors Geert De Schutter, UGent, B Diederik Jacques, SCK•CEN, B Hans Meeussen, NRG, NL Hans van der Sloot, ECN, NL Tom van Gerven, KU Leuven, B Lian Wang, SCK•CEN, B International Steering Committee Urs Berner, PSI, CH Céline Cau Dit Coumes, CEA, F Frederic Glasser, Aberdeen University, UK Bernd Grambow, SUBATECH, F James Kirkpatrick, Michigan State University, USA Barbara Lothenbach, Empa, CH André Nonat, Univ. Bourgogne, F © SCK•CEN Studiecentrum voor Kernenergie Centre d’Etude de l’Energie Nucléaire Boeretang 200 BE-2400 MOL Belgium http://www.sckcen.be Status: Unclassified RESTRICTED All property rights and copyright are reserved. Any communication or reproduction of this document, and any communication or use of its content without explicit authorization is prohibited. Any infringement to this rule is illegal and entitles to claim damages from the infringer, without prejudice to any other right in case of granting a patent or registration in the field of intellectual property. Copyright of the individual abstracts and papers rests with the authors and the SCK•CEN takes no responsability on behalf of their content and their further use. -
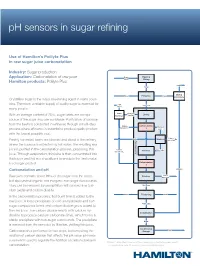
Ph Sensors in Sugar Refining
pH sensors in sugar refining Use of Hamilton’s Polilyte Plus in raw sugar juice carbonatation Industry: Sugar production Application: Carbonatation of raw juice Sugar Washing Beets Slicing Hamilton products: Polilyte Plus Pellets Slices Drying Water Extraction Scraps Pelletation Crystalline sugar is the major sweetening agent in many coun- Lime tries. Therefore, a reliable supply of quality sugar is essential for Raw Juice CaCO3 many people. Lime Lime milk Ca(OH) Liming With an average content of 20%, sugar beets are a major burning 2 source of the sugar sucrose worldwide. Purification of sucrose from the beets is conducted in refineries through a multi-step Carbon Carbonatation Washing dioxide CO process where efficiency is essential to produce quality product 2 1 water with the lowest possible cost. Lime + Water Washing Freshly harvested beets are cleaned and sliced at the refinery, impurities g where the sucrose is extracted by hot water. The resulting raw Cleanin juice is purified in the carbonatation process, producing thin Carbon Filtration Filter cake dioxide CO2 juice. Through evaporation, thin juice is then concentrated into Juice thick juice and fed into crystallizers to produce the final crystal- Carbonatation lized sugar product. 2 Carbonatation and pH Melasse Lime + Raw juice contains about 99% of the sugar from the beets, Filtration impurities but also several organic and inorganic non-sugar compounds. They can be removed by precipitation with burned lime (cal- Thin Juice cium oxide) and carbon dioxide. Steam Thickening Steam In the carbonatation process, first burnt lime is added to the raw juice. A loose precipitate of calcium hydroxide and non- Thick Juice sugar compounds forms and carbon dioxide gas is added to the raw juice. -

Carbonation of Alkaline Paper Mill Waste to Reduce CO2 Greenhouse Gas Emissions Into the Atmosphere R
Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas emissions into the atmosphere R. Perez-Lopez, G. Montes-Hernandez, J.-M. Nieto, F. Renard, L. Charlet To cite this version: R. Perez-Lopez, G. Montes-Hernandez, J.-M. Nieto, F. Renard, L. Charlet. Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas emissions into the atmosphere. Applied Geochemistry, Elsevier, 2008, 23 (8), pp.2292 à 2300. 10.1016/j.apgeochem.2008.04.016. insu-00351929 HAL Id: insu-00351929 https://hal-insu.archives-ouvertes.fr/insu-00351929 Submitted on 12 Jan 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas 2 emissions into the atmosphere 3 4 R. Pérez-López∗, a,b, G. Montes-Hernandez a, J.M. Nieto b, F. Renard c,d, L. Charlet a 5 6 a LGIT, CNRS-OSUG-UJF, Université Joseph Fourier, Grenoble I, Maison des Géosciences, BP 53, 7 38041 Grenoble Cedex, France 8 b Department of Geology, University of Huelva, Campus ‘El Carmen’, 21071, Huelva, Spain 9 c LGCA, CNRS-OSUG-UJF, Université Joseph Fourier, Grenoble I, Maison des Géosciences, BP 53, 10 38041 Grenoble Cedex, France 11 d Physics of Geological Processes, University of Oslo, Norway 12 13 14 To be submitted to Applied Geochemistry on December 18th, 2007 15 ∗ Corresponding author. -
May 16, 1967 W. F. WALDECK 3,320,0 6 METHOD of PREPARING CALCITE and the PRODUCT THEREOF Filed Feb
May 16, 1967 w. F. WALDECK 3,320,0 6 METHOD OF PREPARING CALCITE AND THE PRODUCT THEREOF Filed Feb. 20, 1964 5 Sheets-Sheet 1 PRIOR ART (.7, I____: ONE MICRON May 15, 1967 w. F. wALDEcK “ 3,320,026 METHOD OF PREPARING CALCITE vAND THE PRODUCT THEREOF Filed Feb. 20, 1964 5 Sheets-Sheet 2 5%, w M? l__:__.| ONE MICRON May 16, 1967 w. F. WALDECK 3,320,026 METHOD OF PREPARING CALCITE AND THE PRODUCT THEREOF Filed Feb. 20, 1964 5 Sheets-Sheet 5 O.l MICRON Fig.6 3,320,026 United States Patent nice Patented May 16, 1967 1 2 complete reaction to product substantially free of other 3,320,026 crystalline forms. METHDD 0F PREPARING CALCITE AND THE PRODUCT THEREOF Carbonatation may be conducted in standard gas-liquid William F. Waldeclr, Milford, N.J., assignor to Chas. contacting equipment. For example, an agitated tank P?zer & Co., Inc., New York, N.Y., a corporation of may be employed, carbon dioxide-containing gas being Delaware suitably injected beneath the agitator for thorough dis Filed Feb. 20, 1964, Ser. No. 346,349 persion throughout the reaction mixture. A typical plant 16 Claims. (Cl. 23-66) scale precipitation may require one or two hours, under which circumstances it will usually be appropriate to This invention is concerned with calcium carbonate, 10 maintain the temperature below 20° C. for about 10 or and more particularly with a calcite of novel crystal 15 minutes, by recirculating some of the slurry through habit, and processes for its preparation and use. -

CO2 Sequestration in the Production of Portland Cement Mortars with Calcium Carbonate Additions
nanomaterials Article CO2 Sequestration in the Production of Portland Cement Mortars with Calcium Carbonate Additions Marius-George Parvan, Georgeta Voicu *, Alina-Ioana Badanoiu, Adrian-Ionut Nicoara and Eugeniu Vasile Department of Science and Engineering of Oxide Materials and Nanomaterials, Faculty of Applied Chemistry and Material Science, University POLITEHNICA of Bucharest, 1-7 Gh. Polizu Street, District 1, RO-011061 Bucharest, Romania; [email protected] (M.-G.P.); [email protected] (A.-I.B.); [email protected] (A.-I.N.); [email protected] (E.V.) * Correspondence: [email protected] Abstract: The paper presents the obtention and characterization of Portland cement mortars with limestone filler and nano-calcite additions. The nano-calcite was obtained by the injection of CO2 in a nano-Ca(OH)2 suspension. The resulted nano-CaCO3 presents different morphologies, i.e., polyhedral and needle like crystals, depending on the initial Ca(OH)2 concentration of the sus- pension. The formation of calcium carbonate in suspensions was confirmed by X-ray diffraction (XRD), complex thermal analysis (DTA-TG), scanning electron microscopy (SEM) and transmis- sion electron microscopy (TEM and HRTEM). This demonstrates the viability of this method to successfully sequestrate CO2 in cement-based materials. The use of this type of nano-CaCO3 in mortar formulations based on PC does not adversely modify the initial and final setting time of cements; for all studied pastes, the setting time decreases with increase of calcium carbonate content (irrespective of the particle size). Specific hydrated phases formed by Portland cement hydration Citation: Parvan, M.-G.; Voicu, G.; Badanoiu, A.-I.; Nicoara, A.-I.; Vasile, were observed in all mortars, with limestone filler additions or nano-CaCO3, irrespective of curing E. -

Investigation Into the Filterability of Raw Sugars from Different Geographical Regions of the World
International Journal of Computational and Theoretical Chemistry 2017; 5(6): 53-58 http://www.sciencepublishinggroup.com/j/ijctc doi: 10.11648/j.ijctc.20170506.11 ISSN: 2376-7286 (Print); ISSN: 2376-7308 (Online) Investigation into the Filterability of Raw Sugars from Different Geographical Regions of the World Kamal Suleiman Hassan1, Kanada Toto Korea2, Abubakr Musa1, 3, * 1Sugar Institute, University of Gezira, Wad Madani, Algezira, Sudan 2Alkhaleej Sugar Co., Dubai, UAE 3School of Food and Biological Engineering, Jiangsu University, Zhenjiang, China Email address: [email protected] (A. Musa) *Corresponding author To cite this article: Kamal Suleiman Hassan, Kanada Toto Korea, Abubakr Musa. Investigation into the Filterability of Raw Sugars from Different Geographical Regions of the World. International Journal of Computational and Theoretical Chemistry. Vol. 5, No. 6, 2017, pp. 53-58. doi: 10.11648/j.ijctc.20170506.11 Received: October 19, 2017; Accepted: November 8, 2017; Published: January 2, 2018 Abstract: This study was carried out at Alkhaleej Sugar Refinery in Dubai, United Arab Emirates. The study aimed to find out the correlation between the filterability and slurry resistance value so fraw meltliquor in the carbonation sugar refinery and the raw sugar quality. The investigation utilized 5 raw sugar samples originated from various regions of the world comprising three quality groups namely; low pol sugar (LP) originated from Thailand, very high pol sugar (VHP) originated from Brazil, South-Africa, Sudan, India, and very high pol sugar (VVHP) originated from Brazil. The filterability and the slurry resistance tests were used as the main determining factors for the evaluation. The filterability of refined sugar (consideredas100%) was used as a reference value for comparison. -

Performance Evaluation of Precast Reinforced Concrete After Early-Age Carbonation Curing
Performance Evaluation of Precast Reinforced Concrete after Early-Age Carbonation Curing Duo Zhang Department of Civil Engineering and Applied Mechanics McGill University, Montreal July 2016 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Engineering © Duo Zhang, 2016 All Rights Reserved This page is intentionally left blank ABSTRACT Early carbonation curing of precast concrete accelerates early strength and promotes carbon dioxide utilization. Reinforced concrete represents the majority of precast concrete products and thus the application of early-age carbonation technology in reinforced concrete curing is able to enhance the carbon dioxide utilization potential by concrete products for emission reduction and performance improvement. This thesis aims to develop a special carbonation process suitable to high slump precast concrete. The performance of both ordinary Portland cement (OPC) concrete and fly ash-OPC concretes were evaluated. Early carbonation behavior was assessed by carbon uptakes, pH values, compressive strength and process shrinkage. The effect of early carbonation curing on cement reaction, pozzolanic reaction and pore structure was also examined to interpret the mechanisms of durability performance including freeze-thaw resistance, chloride penetration and weathering carbonation. It was found that carbonation curing had reduced both pore volume and pore size. Gel pores became dominant which were responsible for the improved resistance to permeation and freeze-thaw damage. Chemically cement reaction was accelerated in the early age by carbonation curing and concrete pH values could be compensated by subsequent re-hydration. It was concluded that the carbonation curing was suited to the precast reinforced concrete with or without fly ash incorporation in order to gain improved early strength, enhanced durability and permanent carbon dioxide sequestration as an added-value for environmental benefit. -

Synthesis and X-Ray Diffraction Analyses of Calcium Hydroxide Nanoparticles in Aqueous Suspension
Advances in Materials Physics and Chemistry, 2013, 3, 108-112 http://dx.doi.org/10.4236/ampc.2013.31A013 Published Online April 2013 (http://www.scirp.org/journal/ampc) Synthesis and X-Ray Diffraction Analyses of Calcium Hydroxide Nanoparticles in Aqueous Suspension G. Taglieri1, C. Mondelli2, V. Daniele1, E. Pusceddu3, A. Trapananti4 1University of L’Aquila, Department of Industrial and Information Engineering and Economics, L’Aquila, Italy 2CNR-IOM-OGG Institut Laue Langevin, Grenoble, France 3Institut Laue Langevin, Grenoble, France 4CNR-IOM-OGG, c/o ESRF, Grenoble, France Email: [email protected], [email protected], [email protected], [email protected] Received January 24, 2013; revised March 12, 2013; accepted April 17, 2013 Copyright © 2013 G. Taglieri et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Calcium hydroxide nanoparticles in aqueous suspensions (also called nanolime) were successfully employed in Cultural Heritage conservation thanks to the ability of favoring re-adhesion of the pictorial layer on original carbonatic sub- strates or allowing to a better superficial cohesion and protection of treated stones. In this work, we have synthesized nanolime particles in aqueous suspension by two different methods. The produced particles were characterized in the laboratory, in terms of structural and morphological features, by means of X-Ray diffraction powder (XRD) and by transmission electron microscopy (TEM), respectively. Nanoparticles were crystalline, regularly shaped, hexagonally plated and with side dimensions generally ranging from 300 nm to 30 nm or less. -

The Effects of Chemical Purification on the Color of Thin and Thick Juices in Sugar Beet Factories Maryam Arajshirvani1, Mohammad Hojjatoleslami*1
Nutrition and Food Sciences Research Vol 4, No 2, Apr-Jun 2017, pages:35-41 Original Article The Effects of Chemical Purification on the Color of Thin and Thick Juices in Sugar Beet Factories Maryam Arajshirvani1, Mohammad Hojjatoleslami*1 1- Food Sciences Department, Islamic Azad University, Shahrekord Branch, Iran. Received: October 2016 Accepted: January 2017 A B S T R A C T Background and Objectives: Recognizing the impurities of sugar beet and also comparing different methods of juice purification have shown that the chemical purification based on lime and carbon dioxide is really efficient and economic in the sugar industry. Factories have to control and improve purification stages to achieve clear juice with high chemical purity, low color, minimum hardness, sufficient thermal resistance and with no insoluble solid matters in producing white sugar with high purity and efficiency. The aim of this study was to investigate the effects of alkalinity changes in preliming and liming stages on improving the color of thin and thick juices. Materials and Methods: In the current study, changes in pre-liming alkalinity (0.2, 0.3 and 0.9) and liming alkalinity (1.3, 1.4, 1.5 and 1.6) (mg CaO/ 100 ml juice) during the operation were studied in Sharekrod sugar factory. According to the discharge and retention time of each stage (raw juice, thin juice, and thick juice), the samples were taken and the color changes were studied. Results: The results showed that alkalinity changes of 0.4 in preliming along 1.4 in liming and 0.4 in preliming along 1.3 in liming (mg CaO/ 100 ml juice) caused imbalance of cation/anion, sucrose hydrolysis and invert sugar, and produced undesired color in the thin or thick juice. -

Application of Hydrodynamic Oscillations for Activation of the Hydrated Lime Slurry
─── Food Technology ─── Application of hydrodynamic oscillations for activation of the hydrated lime slurry Iryna Dubovkina Institute of Engineering Thermophysics of National Academy of Sciences of Ukraine, Kyiv, Ukraine Abstract Keywords: Introduction. The purpose of this scientific work is to study the impact of the application of hydrodynamic Water oscillations for activation of the hydrated lime slurry in the Hydrated production of sugar from sugar beets. Lime Materials and methods. General scientific methods, Pressure special methods, volume parametric imitation and Oscillation visualization modelling methods, math modelling methods, optical microscopy, and ionometry were used for the researches. Results and discussion. It is established that application Article history: of hydrodynamic oscillations for activation of the hydrated lime slurry in the production of sugar from sugar beets is Received 02.09.2017 very perspective. Received in revised It was established that the value of the linear speeds of a form 13.12.2017 stream should be within 22 m/s for the first rotor and 24 m/s Accepted 29.12.2017 for the second rotor for intensification mass exchange processes between lime and water throughout the activating hydrated lime slurry. Corresponding The research studies demonstrated the increasing of the author: potential of hydrogen of the water prepared for the technology of the activating hydrated lime slurry for the Iryna Dubovkina processes of juice purification within 15%. E-mail: In general case was established that the decreasing of [email protected] reduction-oxidation reaction which obtained throughout processing on an extent 210s, after that there is not large decreasing of the reduction-oxidation reaction. -

Cane Sugar 10 27 2017.Pdf
Cane Sugar Refining with Ion Exchange Resins Table of Contents Cane Sugar Mills 2 Technical Information 28 Physical Constants of Sucrose 28 Cane Sugar Refining Process 4 Ash vs. Conductivity 30 Raw Sugar Receiving 4 Sucrose Viscosity 30 Affination 4 Melter 4 Regenerant Concentrations 34 Defecation/Clarification 4 Filtration 4 Conversions 35 Decolorization 5 Evaporation 5 Mesh and Slot Sizes 37 Crystallization 5 Amorfo Sugar 6 Resin Technical Data 38 A860S 38 Decolorization 7 A502PS 38 Colorants 8 A420S 38 Color Removal Mechanisms 9 MN102 38 Adsorbents 9 MN150 38 Resin Advantages 11 IP4 38 Service 12 C115EC 38 Direction, Loading, Leakage 13 A500SPlus 38 Odor and Taste 15 A850S 38 Regeneration 16 A440S 38 Sequence, Cleanup Regeneration, 17 PRA420 38 Regeneration Without Brine 18 CT124SH 38 Demineralization 21 ICUMSA Color Testing 39 Liquid Sucrose 21 Liquid Invert 22 Cleaning Organically Fouled Resins 40 Nanofiltration Recovery of Regenerants 23 Tank Capacities 41 Lab Testing Protocol 24 Glossary 42 1 Cane Sugar Refining with Ion Exchange Resins Cane Sugar Refining with Ion Exchange Resins Sucrose is extracted from the sugar cane stalk in a cane sugar Mill, purified and crystallized into a tan colored raw sugar. The sugar we consume must be further refined so as to not impart color, flavor or odor to the foods and beverages it sweetens. In a cane sugar Refinery the raw sugar is remelted and the sucrose is further decolorized with ion exchange resins, carbon or bone char and crystallized into white sugar for industry, consumers and confectioners. Although the ion exchange resins are used in the refinery, it is useful to understand the mill operations also.