Opportunities for Geologic Carbon Sequestration in Washington State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Municipal Solid Waste Incineration (MSWI) Ashes for Greener Concrete Master’S Project for the Master Program of Structural
Municipal solid waste incineration (MSWI) ashes for greener concrete Master’s project for the Master Program of Structural Engineering and Building Technology ARNAUD GLIKSON SARA LÓPEZ MENÉNDEZ Department of Architecture and Civil Engineering Division of Building Technology Building Materials CHALMERS UNIVERSITY OF TECHNOLOGY Master’s Thesis ACEX30-19-97 Gothenburg, Sweden 2019 MASTER’S THESIS ACEX30-19-97 Municipal solid waste incineration (MSWI) ashes for greener concrete Master’s project for the Master Program of Structural Engineering and Building Technology ARNAUD GLIKSON SARA LÓPEZ MENÉNDEZ Department of Architecture and Civil Engineering Division of Building Technology Building Materials CHALMERS UNIVERSITY OF TECHNOLOGY Göteborg, Sweden 2019 Municipal solid waste incineration (MSWI) ashes for greener concrete Master’s project for the Master Program of Structural Engineering and Building Technology ARNAUD GLIKSON SARA LÓPEZ MENÉNDEZ © ARNAUD GLIKSON, SARA LÓPEZ MENÉNDEZ, 2019 Examensarbete ACEX30-19-97 Institutionen för arkitektur och samhällsbyggnadsteknik Chalmers tekniska högskola, 2019 Department of Architecture and Civil Engineering Division of Building Technology Building Materials Chalmers University of Technology SE-412 96 Göteborg Sweden Telephone: + 46 (0)31-772 1000 Department of Architecture and Civil Engineering Göteborg, Sweden, 2019 Municipal solid waste incineration (MSWI) ashes for greener concrete Master’s project for the Master Program of Structural Engineering and Building Technology ARNAUD GLIKSON SARA LÓPEZ MENÉNDEZ Department of Architecture and Civil Engineering Division of Building Technology Building Materials Chalmers University of Technology ABSTRACT Owing to the varying characteristics of municipal solid waste incineration (MSWI) ashes and the lack of harmonized standards and regulations, a substantial portion of MSWI ashes are simply used as landfilling cover at the present, which can be better utilized in the viewpoint of sustainability. -

A Review of Natural CO2 Occurrences and Their Relevance to CO2 Storage. British Geological Survey External Report, CR/05/104, 117Pp
A REVIEW OF NATURAL CO2 OCCURRENCES AND RELEASES AND THEIR RELEVANCE TO CO2 STORAGE Report Number 2005/8 September 2005 This document has been prepared for the Executive Committee of the Programme. It is not a publication of the Operating Agent, International Energy Agency or its Secretariat. A REVIEW OF NATURAL CO2 OCCURRENCES AND RELEASES AND THEIR RELEVANCE TO CO2 STORAGE Background to the Study The security of storage of CO2 in geological reservoirs is a key issue that has been, and will be increasingly, discussed as the technology moves closer to wide scale deployment. There are a number of ways through which the security of storage can be demonstrated. These include: • Development of standards and best practise guidelines that ensure that storage reservoirs are carefully selected and the environmental risks are minimised, • Development of risk assessment procedures that demonstrate long term safe storage is a realistic prospect, • Monitoring of injection projects to confirm the containment of injected CO2. All these activities are now underway in a number of countries worldwide. However it may be several more years before a credible data base is established that will allow the issue of security of storage to be resolved to everybody’s satisfaction. In the intervening period, this issue will represent a potential barrier to the introduction of CO2 storage technology. In that interim period, groups not necessarily supportive of geological storage of CO2, may emphasise the issue of storage security, through reference to natural geological events. In particular, natural events such as Lake Nyos in Cameroon that have resulted in deaths due to an uncontrolled CO2 release may well be those that are focused upon in any debate. -

Oil and Gas Industry Engagement on Climate Change Drivers, Actions, and Path Forward
OCTOBER 2019 Oil and Gas Industry Engagement on Climate Change Drivers, Actions, and Path Forward AUTHOR Stephen Naimoli Sarah Ladislaw A Report of the CSIS ENERGY AND NATIONAL SECURITY PROGRAM OCTOBER 2019 Oil and Gas Industry Engagement on Climate Change Drivers, Actions, and Path Forward AUTHORS Stephen Naimoli Sarah Ladislaw A Report of the CSIS Energy and National Security Program About CSIS Established in Washington, D.C., over 50 years ago, the Center for Strategic and International Studies (CSIS) is a bipartisan, nonprofit policy research organization dedicated to providing strategic in sights and policy solutions to help decisionmakers chart a course toward a better world. In late 2015, Thomas J. Pritzker was named chairman of the CSIS Board of Trustees. Mr. Pritzker succeeded former U.S. senator Sam Nunn (D-GA), who chaired the CSIS Board of Trustees from 1999 to 2015. CSIS is led by John J. Hamre, who has served as president and chief executive officer since 2000. Founded in 1962 by David M. Abshire and Admiral Arleigh Burke, CSIS is one of the world’s preeminent international policy in stitutions focused on defense and security; regional study; and transnational challenges ranging from energy and trade to global development and economic integration. For eight consecutive years, CSIS has been named the world’s number one think tank for defense and national security by the University of Pennsylvania’s “Go To Think Tank Index.” The Center’s over 220 full-time staff and large network of affiliated scholars conduct research and analysis and develop policy initiatives that look to the future and anticipate change. -

BOOK of ABSTRACTS SCK•CEN-BA-53 13/Dja/P-23
BOOK OF ABSTRACTS SCK•CEN-BA-53 13/Dja/P-23 3rd International Workshop Mechanisms and modelling of waste/cement interactions Ghent, 06-08 May 2013 SCK•CEN Boeretang 200 BE-2400 MOL Belgium http://www.sckcen.be BOOK OF ABSTRACTS SCK•CEN-BA-53 3rd International Workshop Mechanisms and modelling of waste/cement interactions Ghent, 06-08 May 2013 SCK•CEN, Boeretang 200, BE-2400 MOL, Belgium Contact: Diederik Jacques Tel: +32 14 33 32 09 E-mail: [email protected] Organising committee Workshop convenors Geert De Schutter, UGent, B Diederik Jacques, SCK•CEN, B Hans Meeussen, NRG, NL Hans van der Sloot, ECN, NL Tom van Gerven, KU Leuven, B Lian Wang, SCK•CEN, B International Steering Committee Urs Berner, PSI, CH Céline Cau Dit Coumes, CEA, F Frederic Glasser, Aberdeen University, UK Bernd Grambow, SUBATECH, F James Kirkpatrick, Michigan State University, USA Barbara Lothenbach, Empa, CH André Nonat, Univ. Bourgogne, F © SCK•CEN Studiecentrum voor Kernenergie Centre d’Etude de l’Energie Nucléaire Boeretang 200 BE-2400 MOL Belgium http://www.sckcen.be Status: Unclassified RESTRICTED All property rights and copyright are reserved. Any communication or reproduction of this document, and any communication or use of its content without explicit authorization is prohibited. Any infringement to this rule is illegal and entitles to claim damages from the infringer, without prejudice to any other right in case of granting a patent or registration in the field of intellectual property. Copyright of the individual abstracts and papers rests with the authors and the SCK•CEN takes no responsability on behalf of their content and their further use. -
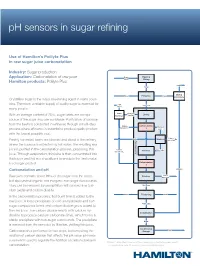
Ph Sensors in Sugar Refining
pH sensors in sugar refining Use of Hamilton’s Polilyte Plus in raw sugar juice carbonatation Industry: Sugar production Application: Carbonatation of raw juice Sugar Washing Beets Slicing Hamilton products: Polilyte Plus Pellets Slices Drying Water Extraction Scraps Pelletation Crystalline sugar is the major sweetening agent in many coun- Lime tries. Therefore, a reliable supply of quality sugar is essential for Raw Juice CaCO3 many people. Lime Lime milk Ca(OH) Liming With an average content of 20%, sugar beets are a major burning 2 source of the sugar sucrose worldwide. Purification of sucrose from the beets is conducted in refineries through a multi-step Carbon Carbonatation Washing dioxide CO process where efficiency is essential to produce quality product 2 1 water with the lowest possible cost. Lime + Water Washing Freshly harvested beets are cleaned and sliced at the refinery, impurities g where the sucrose is extracted by hot water. The resulting raw Cleanin juice is purified in the carbonatation process, producing thin Carbon Filtration Filter cake dioxide CO2 juice. Through evaporation, thin juice is then concentrated into Juice thick juice and fed into crystallizers to produce the final crystal- Carbonatation lized sugar product. 2 Carbonatation and pH Melasse Lime + Raw juice contains about 99% of the sugar from the beets, Filtration impurities but also several organic and inorganic non-sugar compounds. They can be removed by precipitation with burned lime (cal- Thin Juice cium oxide) and carbon dioxide. Steam Thickening Steam In the carbonatation process, first burnt lime is added to the raw juice. A loose precipitate of calcium hydroxide and non- Thick Juice sugar compounds forms and carbon dioxide gas is added to the raw juice. -

Carbonation of Alkaline Paper Mill Waste to Reduce CO2 Greenhouse Gas Emissions Into the Atmosphere R
Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas emissions into the atmosphere R. Perez-Lopez, G. Montes-Hernandez, J.-M. Nieto, F. Renard, L. Charlet To cite this version: R. Perez-Lopez, G. Montes-Hernandez, J.-M. Nieto, F. Renard, L. Charlet. Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas emissions into the atmosphere. Applied Geochemistry, Elsevier, 2008, 23 (8), pp.2292 à 2300. 10.1016/j.apgeochem.2008.04.016. insu-00351929 HAL Id: insu-00351929 https://hal-insu.archives-ouvertes.fr/insu-00351929 Submitted on 12 Jan 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas 2 emissions into the atmosphere 3 4 R. Pérez-López∗, a,b, G. Montes-Hernandez a, J.M. Nieto b, F. Renard c,d, L. Charlet a 5 6 a LGIT, CNRS-OSUG-UJF, Université Joseph Fourier, Grenoble I, Maison des Géosciences, BP 53, 7 38041 Grenoble Cedex, France 8 b Department of Geology, University of Huelva, Campus ‘El Carmen’, 21071, Huelva, Spain 9 c LGCA, CNRS-OSUG-UJF, Université Joseph Fourier, Grenoble I, Maison des Géosciences, BP 53, 10 38041 Grenoble Cedex, France 11 d Physics of Geological Processes, University of Oslo, Norway 12 13 14 To be submitted to Applied Geochemistry on December 18th, 2007 15 ∗ Corresponding author. -

Researcher, Environmental Defense Fund Shanda Fisher, Researcher, Environmental Defense Fund
Policy Recommendations for Selection & Development of Offshore Geologic Carbon Sequestration Projects Within Texas State Waters Gulf of Mexico Miocene CO2 Site Characterization Mega Transect: Environmental Risks and Regulatory Considerations for Site Selection December 2, 2011 Principal Authors: Timothy O’Connor, Director, California Climate and Energy Initiative, Environmental Defense Fund Scott Anderson, Senior Energy Advisor, Environmental Defense Fund Daniel Carlin, Researcher, Environmental Defense Fund Shanda Fisher, Researcher, Environmental Defense Fund Abstract: This report evaluates the potential environmental impact of geologic carbon sequestration projects in the state waters of Texas and makes recommendations for decisions that can be followed during the site selection phase to alleviate risk and mitigate potential harm. This report also makes related recommendations for consideration during the project development and operations phase related to site-specific monitoring, verification, accounting and reporting, and response planning. Gulf of Mexico Miocene CO2 Site Characterization Mega Transect: Environmental Risks and Regulatory Considerations for Site Selection DISCLAIMER Environmental Defense Fund (EDF) prepared this report to support the University of Texas Bureau of Economic Geology’s Gulf of Mexico Miocene CO2 Site Characterization Mega Transect project, related to identifying and choosing a suitable sequestration site or site(s), and as funded by the U.S. Department of Energy. This report is intended to serve as a -

Carbon Capture in Texas: Comparative Advantage in a Low Carbon Portfolio
Carbon Capture, Hydrogen and Collaborative Action to Reduce Industrial Emissions in Texas CARBON CAPTURE IN TEXAS: COMPARATIVE ADVANTAGE IN A LOW CARBON PORTFOLIO Kenneth B. Medlock III, Ph.D. James A. Baker, III, and Susan G. Baker Fellow in Energy and Resource Economics and Senior Director, Center for Energy Studies Keily Miller Research Manager, Center for Energy Studies June 2020 © 2020 Rice University’s Baker Institute for Public Policy This material may be quoted or reproduced without prior permission, provided appropriate credit is given to the authors and Rice University’s Baker Institute for Public Policy. Wherever feasible, papers are reviewed by outside experts before they are released. However, the research and views expressed in this paper are those of the individual researcher(s) and do not necessarily represent the views of the Baker Institute. Kenneth B. Medlock III, Ph.D. Keily Miller “Carbon Capture in Texas: Comparative Advantage in a Low Carbon Portfolio” https://doi.org/10.25613/9ffx-6h23 Carbon Capture in Texas: Comparative Advantage in a Low Carbon Portfolio Introduction World economic growth will drive future demands for energy. The International Monetary Fund’s World Economic Outlook (April 2020) predicts that emerging and developing economies will account for the majority of global economic growth through the 2020s, with China and India leading the way. In general, as human and industrial activity expands, the concomitant growth in energy consumption introduces a complex paradigm. Achieving the dual goals of economic and environmental sustainability will be among the world's most pressing challenges, and the burden will not be evenly distributed. -
May 16, 1967 W. F. WALDECK 3,320,0 6 METHOD of PREPARING CALCITE and the PRODUCT THEREOF Filed Feb
May 16, 1967 w. F. WALDECK 3,320,0 6 METHOD OF PREPARING CALCITE AND THE PRODUCT THEREOF Filed Feb. 20, 1964 5 Sheets-Sheet 1 PRIOR ART (.7, I____: ONE MICRON May 15, 1967 w. F. wALDEcK “ 3,320,026 METHOD OF PREPARING CALCITE vAND THE PRODUCT THEREOF Filed Feb. 20, 1964 5 Sheets-Sheet 2 5%, w M? l__:__.| ONE MICRON May 16, 1967 w. F. WALDECK 3,320,026 METHOD OF PREPARING CALCITE AND THE PRODUCT THEREOF Filed Feb. 20, 1964 5 Sheets-Sheet 5 O.l MICRON Fig.6 3,320,026 United States Patent nice Patented May 16, 1967 1 2 complete reaction to product substantially free of other 3,320,026 crystalline forms. METHDD 0F PREPARING CALCITE AND THE PRODUCT THEREOF Carbonatation may be conducted in standard gas-liquid William F. Waldeclr, Milford, N.J., assignor to Chas. contacting equipment. For example, an agitated tank P?zer & Co., Inc., New York, N.Y., a corporation of may be employed, carbon dioxide-containing gas being Delaware suitably injected beneath the agitator for thorough dis Filed Feb. 20, 1964, Ser. No. 346,349 persion throughout the reaction mixture. A typical plant 16 Claims. (Cl. 23-66) scale precipitation may require one or two hours, under which circumstances it will usually be appropriate to This invention is concerned with calcium carbonate, 10 maintain the temperature below 20° C. for about 10 or and more particularly with a calcite of novel crystal 15 minutes, by recirculating some of the slurry through habit, and processes for its preparation and use. -

Carbon Dioxide Storage: Geological Security and Environmental Issues – Case Study on the Sleipner Gas Field in Norway
Carbon Dioxide Storage: Geological Security and Environmental Issues – Case Study on the Sleipner Gas field in Norway (Figure courtesy of Statoil) Semere Solomon Bellona Report May 2007 2 Table of contents Table of contents................................................................................................ 1 Executive Summary, Conclusions and Recommendations.................................. 3 1 Introduction................................................................................................. 7 1.1 Background.............................................................................................................7 1.2 Properties of CO2 and Health Effects......................................................................9 1.3 Sources of CO 2......................................................................................................11 1.4 CO 2 Capture and Storage ......................................................................................11 1.5 Context for CO 2 capture and Storage.....................................................................12 1.6 Potential for reducing CO 2 Emissions....................................................................12 1.7 Layout of the Report .............................................................................................13 2 Geological Framework .............................................................................. 15 2.1 Historical perspectives ..........................................................................................15 -

CO2 Sequestration in the Production of Portland Cement Mortars with Calcium Carbonate Additions
nanomaterials Article CO2 Sequestration in the Production of Portland Cement Mortars with Calcium Carbonate Additions Marius-George Parvan, Georgeta Voicu *, Alina-Ioana Badanoiu, Adrian-Ionut Nicoara and Eugeniu Vasile Department of Science and Engineering of Oxide Materials and Nanomaterials, Faculty of Applied Chemistry and Material Science, University POLITEHNICA of Bucharest, 1-7 Gh. Polizu Street, District 1, RO-011061 Bucharest, Romania; [email protected] (M.-G.P.); [email protected] (A.-I.B.); [email protected] (A.-I.N.); [email protected] (E.V.) * Correspondence: [email protected] Abstract: The paper presents the obtention and characterization of Portland cement mortars with limestone filler and nano-calcite additions. The nano-calcite was obtained by the injection of CO2 in a nano-Ca(OH)2 suspension. The resulted nano-CaCO3 presents different morphologies, i.e., polyhedral and needle like crystals, depending on the initial Ca(OH)2 concentration of the sus- pension. The formation of calcium carbonate in suspensions was confirmed by X-ray diffraction (XRD), complex thermal analysis (DTA-TG), scanning electron microscopy (SEM) and transmis- sion electron microscopy (TEM and HRTEM). This demonstrates the viability of this method to successfully sequestrate CO2 in cement-based materials. The use of this type of nano-CaCO3 in mortar formulations based on PC does not adversely modify the initial and final setting time of cements; for all studied pastes, the setting time decreases with increase of calcium carbonate content (irrespective of the particle size). Specific hydrated phases formed by Portland cement hydration Citation: Parvan, M.-G.; Voicu, G.; Badanoiu, A.-I.; Nicoara, A.-I.; Vasile, were observed in all mortars, with limestone filler additions or nano-CaCO3, irrespective of curing E. -

A Review of Developments in Carbon Dioxide Storage
A Review of Developments in Carbon Dioxide Storage Mohammed D. Aminua, Christopher A. Rochelleb, Seyed Ali Nabavia, Vasilije Manovica,* a Combustion, Carbon Capture and Storage Centre, Cranfield University, Bedford, Bedfordshire, MK43 0AL, UK b British Geological Survey, Environmental Science Centre, Nicker Hill, Keyworth, Nottingham NG12 5GG, UK *Corresponding author: Email: [email protected], Tel: +44(0)1234754649 Abstract Carbon capture and storage (CCS) has been identified as an urgent, strategic and essential approach to reduce anthropogenic CO2 emissions, and mitigate the severe consequences of climate change. CO2 storage is the last step in the CCS chain and can be implemented mainly through oceanic and underground geological sequestration, and mineral carbonation. This review paper aims to provide state-of-the-art developments in CO2 storage. The review initially discussed the potential options for CO2 storage by highlighting the present status, current challenges and uncertainties associated with further deployment of established approaches (such as storage in saline aquifers and depleted oil and gas reservoirs) and feasibility demonstration of relatively newer storage concepts (such as hydrate storage and CO2-based enhanced geothermal systems). The second part of the review outlined the critical criteria that are necessary for storage site selection, including geological, geothermal, geohazards, hydrodynamic, basin maturity, and economic, societal and environmental factors. In the third section, the focus was on identification of CO2 behaviour within the reservoir during and after injection, namely injection-induced seismicity, potential leakage pathways, and long-term containment complexities associated with CO2-brine-rock interaction. In addition, a detailed review on storage capacity estimation methods based on different geological media and trapping mechanisms was provided.