Foot and Ankle Functional Morphology in Anthropoid Primates and Miocene Hominoids
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Unravelling the Positional Behaviour of Fossil Hominoids: Morphofunctional and Structural Analysis of the Primate Hindlimb
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Doctorado en Biodiversitat Facultad de Ciènces Tesis doctoral Unravelling the positional behaviour of fossil hominoids: Morphofunctional and structural analysis of the primate hindlimb Marta Pina Miguel 2016 Memoria presentada por Marta Pina Miguel para optar al grado de Doctor por la Universitat Autònoma de Barcelona, programa de doctorado en Biodiversitat del Departamento de Biologia Animal, de Biologia Vegetal i d’Ecologia (Facultad de Ciències). Este trabajo ha sido dirigido por el Dr. Salvador Moyà Solà (Institut Català de Paleontologia Miquel Crusafont) y el Dr. Sergio Almécija Martínez (The George Washington Univertisy). Director Co-director Dr. Salvador Moyà Solà Dr. Sergio Almécija Martínez A mis padres y hermana. Y a todas aquelas personas que un día decidieron perseguir un sueño Contents Acknowledgments [in Spanish] 13 Abstract 19 Resumen 21 Section I. Introduction 23 Hominoid positional behaviour The great apes of the Vallès-Penedès Basin: State-of-the-art Section II. Objectives 55 Section III. Material and Methods 59 Hindlimb fossil remains of the Vallès-Penedès hominoids Comparative sample Area of study: The Vallès-Penedès Basin Methodology: Generalities and principles Section IV. -
Cambridge University Press 978-1-108-45600-5 — Assembly of the Executive Mind Michael W
Cambridge University Press 978-1-108-45600-5 — Assembly of the Executive Mind Michael W. Hoffmann Index More Information Index abaragnosis, 35 Alvarez bolide impact theory, anosognosia, 196, 198–199 abstract art 21 Antarctic ice sheet response to, 207–208 Alzheimer’s disease, 10, 70, periods of expansion, 36 abstract thought, 58 71, 82, 102, 126, 128, 146, Antarctica, 19, 30, 32 abulia, 54, 91 154, 201, 209, 214 anterior cingulate circuit, 54 Acanthostega, 18 acetylcholine deficit, 78 anthropoids, 36 acetylcholine, 53, 78, 146, 147 amyloid hypothesis, 140 definition, 36 effects of depletion, 146 amyloid-β accumulation antisocial personality disorder, functions and evolutionary and sleep disturbance, 56 role, 79 157 anxiety action observation treatment, default mode network evolution of the mammalian 182 (DMN) and, 127 fear module, 22–23 Adapidae, 29 music therapy, 175 anxiety disorders, 56 addictions, 192 network dysfunction and, apathy, 54, 91 addictive behavior 62 ape–monkey divergence, 36 dopamine and the reward vascular dysregulation aphasia, 70, 97, 98, 201 pathway, 197–198 hypothesis, 140 Apollo 11 cave, Namibia, 110 Aegyptopithecus, 36, 37 amantadine, 10, 198 apotemnophilia, 199 Afar basin, 60 for severe TBI, 182–183 apraxia, 28, 98 Africa American football aqua–arboreal phase, 195 early primate species, 36–38 repetitive brain injury and aqua–arboreal theory of Miocene fossils, 36–37 CTE, 131–132 bipedalism, 39–40 African Middle Stone Age amino acids, 15, 16 dietary and nutritional (AMSA), 110 amoebae factors, 44–45 African Rift -

The Effect of Loading, Plantar Ligament Disruption and Surgical
The Effect of Loading, Plantar Ligament Disruption and Surgical Repair on Canine Tarsal Bone Kinematics Presented by Christopher John Tan Sydney School of Veterinary Science Faculty of Science, The University of Sydney February 2018 A thesis submitted to fulfil requirements for the degree of Doctor of Philosophy i To my wonderful family i This is to certify that to the best of my knowledge, the content of this thesis is my own work. This thesis has not been submitted for any degree or other purposes. I certify that the intellectual content of this thesis is the product of my own work and that all the assistance received in preparing this thesis and sources have been acknowledged. Signature Name: Christopher John Tan ii Table of contents Statement of originality……………………………………………………………………………………………………………………ii Table of figures……………………………………………………………………………………………………………………………….vii Table of tables………………………………………………………………………………………………………………………………..xiii Table of equations………………………………………………………………………………………………………………………….xvi Abbreviations…………………………………………………………………………………………………………………………………xvii Author Attribution Statement and published works…………………………………………………………………….xviii Summary…………………………………………………………………………………………………………………………………………xix Preface…………………………………………………………………………………………………………………………………………….xx Chapter 1 Introduction ........................................................................................................................... 1 1.1 Overview ...................................................................................................................................... -

Curriculum Vitae
Curriculum vitae PERSONAL INFORMATION Thomas Alfred Püschel Oxford (United Kingdom) (+44) 7476608464 [email protected] EDUCATION AND TRAINING Jan 2014–Jan 2018 Ph.D. in Adaptive Organismal Biology The University of Manchester, School of Earth and Environmental Sciences (United Kingdom) Dissertation: "Morpho-functional analyses of the primate skeleton: Applying 3D geometric morphometrics, finite element analysis and phylogenetic comparative methods to assess ecomorphological questions in extant and extinct anthropoids." Supervisor: Prof. William Sellers Co-supervisor: Prof. Christian Peter Klingenberg Sep 2012–Aug 2013 M.Sc. in Human Evolution with Distinction The Univerity of York, Hull York Medical School (United Kingdom) Dissertation: "Biomechanical modelling of human femora: A comparison between agriculturalists and hunter-gatherers using finite element analysis, geometric morphometrics and beam theory." Supervisor: Prof. Paul O’Higgins 2007–2011 B.Sc. in Anthropology, Major in Physical Anthropology with Distinction Universidad de Chile, Santiago (Chile) Dissertation: "Deformación intencional del cráneo en los oasis de San Pedro de Atacama: un enfoque morfométrico geométrico." Supervisor: Prof. Germán Manríquez WORK EXPERIENCE 1 Nov 2018–Present Postdoctoral Research Fellow - Leverhulme Early Career Fellowship School of Anthropology and Museum Ethnography, University of Oxford, Oxford (United Kingdom) Postdoctoral fellowship awarded to undertake the project: 'This study has teeth: exploring human origins and Climate Change through time". 3D data collection and application of machine-learning regression techniques to generate ecometric models for East Africa using mammalian teeth. Apr 2018–Oct 2018 Postdoctoral Research Associate School of Earth and Environmental Sciences, Faculty of Science and Engineering, University of Manchester, Manchester (United Kingdom) NERC project 'The co-evolution of human hands and tool using behaviour' led by Prof. -

Foot and Ankle Pain
Foot and Ankle Pain Pain in the ankle and foot may arise from the bones and joints, periarticular soft tissues, nerve roots and peripheral nerves, or vascular structures or referred from the lumbar spine or knee joint. Precise diagnosis of ankle and foot pain rests on a careful history, a thorough examination and a few rationally selected diagnostic tests. The foot can be divided into three sections: hindfoot, midfoot, and forefoot. The hindfoot consists of the calcaneus and talus. The anterior two thirds of the calcaneus articulates with the talus, and the posterior third forms the heel. Medially the sustentaculum tali supports the talus and is joined to the navicular bone by the spring ligament. The talus articulates with the tibia and fibula above at the ankle joint, with the calcaneus below at the subtalar joint, and with the navicular in front at the talonavicular joint. Five tarsal bones make up the midfoot: navicular medially, cuboid laterally and the three cuneiforms distally. The midfoot is separated from the hindfoot by the mid- or transverse tarsal joint (talonavicular and calcaneocuboid articulations), and from the forefoot by the tarsometarsal joints. The forefoot comprises the metatarsals and phalanges. The great toe has two phalanges and two sesamoids embedded in the plantar ligament under the metatarsal head. Each of the other toes has three phalanges. The distal tibiofibular joint is a fibrous joint or syndesmosis between the distal ends of the tibia and fibula. The joint only allows slight malleolar separation on full dorsiflexion of the ankle. Section 21 Page 1 The ankle or talocrural joint is a hinge joint between the distal ends of the tibia and fibula and the trochlea of the talus. -
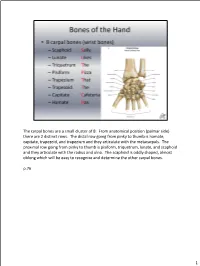
The Carpal Bones Are a Small Cluster of 8. from Anatomical Position (Palmar Side) There Are 2 Distinct Rows
The carpal bones are a small cluster of 8. From anatomical position (palmar side) there are 2 distinct rows. The distal row going from pinky to thumb is hamate, capitate, trapezoid, and trapezium and they articulate with the metacarpals. The proximal row going from pinky to thumb is pisiform, triquetrum, lunate, and scaphoid and they articulate with the radius and ulna. The scaphoid is oddly shaped, almost oblong which will be easy to recognize and determine the other carpal bones. p.76 1 Radiocarpal ligaments (palmar and dorsal)--Connects radius to carpal bones Intercarpal ligaments (palmar and dorsal)--Connects carpal bones to each other Collateral ligaments (ulnar and radial)--Ulnar collateral ligament and radial collateral ligament connect the forearm to the wrist and help support the sides of the wrist as well There are radiocarpal ligaments and intercarpal ligaments on both the dorsal and palmar sides of the hand. p.78 2 So for the middle phalanges there are only #2-5 since the thumb does not have a middle phalange. The proximal phalange and middle phalange articulation is called the proximal interphalangeal joint and the middle phalange articulating with the distal phalange is called the distal interphalangeal joint. With the thumb there is only 1 interphalangeal joint since it’s just the proximal and distal phalange. p.77 3 p.77 4 The radiocarpal joint is where the carpals articulate with the radius (condyloid joint). The intercarpal joints are the gliding joints between the carpals. The 1st carpometacarpal joint is the thumb, which is a saddle joint, but the 2nd-5th carpometacarpal joints are gliding. -

Articulations
9 Articulations PowerPoint® Lecture Presentations prepared by Jason LaPres Lone Star College—North Harris © 2012 Pearson Education, Inc. 9-1 Classification of Joints • Functional Classifications • Synarthrosis (immovable joint) • Amphiarthrosis (slightly movable joint) • Diarthrosis (freely movable joint) © 2012 Pearson Education, Inc. 9-1 Classification of Joints • Synovial Joints (Diarthroses) • Also called movable joints • At ends of long bones • Within articular capsules • Lined with synovial membrane © 2012 Pearson Education, Inc. 9-2 Synovial Joints • Articular Cartilages • Pad articulating surfaces within articular capsules • Prevent bones from touching • Smooth surfaces lubricated by synovial fluid • Reduce friction © 2012 Pearson Education, Inc. 9-2 Synovial Joints • Synovial Fluid • Contains slippery proteoglycans secreted by fibroblasts • Functions of synovial fluid 1. Lubrication 2. Nutrient distribution 3. Shock absorption © 2012 Pearson Education, Inc. 9-2 Synovial Joints • Accessory Structures • Cartilages • Fat pads • Ligaments • Tendons • Bursae © 2012 Pearson Education, Inc. 9-2 Synovial Joints • Cartilages • Cushion the joint • Fibrocartilage pad called a meniscus (or articular disc; plural, menisci) • Fat Pads • Superficial to the joint capsule • Protect articular cartilages • Ligaments • Support, strengthen joints • Sprain – ligaments with torn collagen fibers © 2012 Pearson Education, Inc. 9-2 Synovial Joints • Tendons • Attach to muscles around joint • Help support joint • Bursae • Singular, bursa, a pouch • Pockets -

Gross Anatomy of the Lower Limb. Knee and Ankle Joint. Walking
Gross anatomy of the lower limb. Knee and ankle joint. Walking. Sándor Katz M.D.,Ph.D. Knee joint type: trochoginglimus (hinge and pivot) Intracapsular ligaments: • Anterior cruciate lig. • Posterior cruciate lig. • Transverse lig. • Posterior meniscofemoral .lig. Medial meniscus: C- shaped. Lateral meniscus: almost a complete ring. Knee joint Extracapsular ligaments. Tibial collateral lig. is broader and fuses with the articular capsule and medial meniscus. Fibular collateral lig. is cord-like and separates from the articular capsule. Knee joint - extracapsular ligaments Knee joint - bursae Knee joint - movements • Flexion: 120-130° • Hyperextension: 5° • Voluntary rotation: 50-60° • Terminal rotation: 10° Ankle (talocrural) joint type: hinge Talocrural joint - medial collateral ligament Medial collateral = deltoid ligament Tibionavicular part (1) (partly covers the anterior tibiotalar part) Tibiocalcaneal part (2-3) Posterior tibiotalar part (4) Medial process (6) Sustentaculum tali (7) Tendon of tibialis posterior muscles (9) Talocrural joint - lateral collateral ligament Lateral collateral ligament Anterior talofibular ligament (5, 6) Calcaneofibular ligament (10) Lateral malleolus (1) Tibia (2) Syndesmosis tibiofibularis (3, 4) Talus (7) Collum tali (8) Caput tali (9) Interosseous talocalcaneal ligament (11) Cervical ligament (12) Talonavicular ligament (13) Navicular bone (14) Lateral collateral ligament Posterior talofibular ligament (5) Fibula (1) Tibia (2) Proc. tali, tuberculum laterale (3) Proc. tali, tuberculum mediale (11) Tendo, musculus felxor hallucis longus (8) Lig. calcaneofibulare (12) Tendo, musculus peroneus brevis (13) Tendo, musculus peroneus longus (14) Art. subtalaris (15) Talocrural joint - movements Dorsiflexion: 15° Plantarfelxion: 40° Talotarsal joint (lower ankle joint): talocalcaneonavicular joint and subtalar joint Bony surfaces: anterior and middle talar articular surfaces and head of the talus + anterior and middle calcaneal articular surfaces, navicular. -

Ligamentous Lisfranc Injuries in the Athlete Craig Thomas Haytmanek Jr., MD, and Thomas O
Ligamentous Lisfranc Injuries in the Athlete Craig Thomas Haytmanek Jr., MD, and Thomas O. Clanton, MD Ligamentous injuries to the midfoot during athletic endeavors are becoming more common and more troublesome as they can take significant time before the athlete is able to return to play. Late changes in alignment or posttraumatic arthritis are complications of inadequate treatment. The mechanism of injury is either direct impact to the dorsal midfoot or a twisting injury to the hindfoot with a plantar-flexed, fixed forefoot. Examination reveals ecchymosis and pain in the midfoot. Rarely is there enough instability to allow detection on physical examination. Provocative tests such as external rotation stress of the midfoot or physical activity (single leg hop or walking on tip toes) can recreate symptoms if the patient’spain allows for it. Weight-bearing anteroposterior and lateral radiographic examination of both feet focusing on the midfoot is essential, allowing comparison between the injured and uninjured extremity. Diastasis between the proximal first and second metatarsal is a classic radiographic finding, but proximal extension between the cuneiforms can also be present. A more severe injury shows loss of the longitudinal arch or subluxation of the midfoot that is identified on a lateral radiograph. A tear or an avulsion of Lisfranc ligament along with other midfoot ligaments is the underlying pathology. Advanced imaging modalities including computed tomography and magnetic resonance imaging are useful in these more subtle injuries or when more specific anatomical detail is required. Nondisplaced injuries are typically treated conserva- tively with a period of non–weight bearing followed by a gradual return-to-play protocol. -

Evolution and Function of the Hominin Forefoot
Evolution and function of the hominin forefoot Peter J. Fernándeza,b,1, Carrie S. Monglec, Louise Leakeyd,e, Daniel J. Proctorf, Caley M. Orrg,h, Biren A. Pateli,j, Sergio Almécijak,l,m, Matthew W. Tocherin,o, and William L. Jungersa,p aDepartment of Anatomical Sciences, Stony Brook University, Stony Brook, NY 11794; bDepartment of Biomedical Sciences, Marquette University, Milwaukee, WI 53202; cInterdepartmental Doctoral Program in Anthropological Sciences, Stony Brook University, Stony Brook, NY 11794; dDepartment of Anthropology, Stony Brook University, Stony Brook, NY 11794; eTurkana Basin Institute, Stony Brook University, Stony Brook, NY 11794; fDepartment of Anthropology, Lawrence University, Appleton, WI 54911; gDepartment of Cell and Developmental Biology, University of Colorado School of Medicine, Aurora, CO 80045; hDepartment of Anthropology, University of Colorado Denver, Denver, CO 80204; iDepartment of Integrative Anatomical Sciences, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033; jHuman and Evolutionary Biology Section, Department of Biological Sciences, University of Southern California, Los Angeles, CA 90089; kDivision of Anthropology, American Museum of Natural History, New York, NY 10024; lCenter for the Advanced Study of Human Paleobiology, Department of Anthropology, The George Washington University, Washington, DC 20052; mInstitut Català de Paleontologia Miquel Crusafont (ICP), Universitat Autònoma de Barcelona, 08193 Cerdanyola del Vallès, Barcelona, Spain; nDepartment of Anthropology, Lakehead University, Thunder Bay, ON P7B 5E1, Canada; oHuman Origins Program, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013; and pAssociation Vahatra, Antananarivo 101, Madagascar Edited by Bruce Latimer, Case Western Reserve University, Cleveland, OH, and accepted by Editorial Board Member C. O. Lovejoy July 12, 2018 (received for review January 15, 2018) The primate foot functions as a grasping organ. -

Sigma Gamma Epsilon Student Research Poster Session, Geological Society of America Meeting 2017, Seattle, Washington, Usa
The Compass: Earth Science Journal of Sigma Gamma Epsilon Volume 89 Issue 2 Article 1 4-3-2018 SIGMA GAMMA EPSILON STUDENT RESEARCH POSTER SESSION, GEOLOGICAL SOCIETY OF AMERICA MEETING 2017, SEATTLE, WASHINGTON, USA Paula Even University of Northern Iowa, [email protected] Follow this and additional works at: https://digitalcommons.csbsju.edu/compass Part of the Earth Sciences Commons Recommended Citation Even, Paula (2018) "SIGMA GAMMA EPSILON STUDENT RESEARCH POSTER SESSION, GEOLOGICAL SOCIETY OF AMERICA MEETING 2017, SEATTLE, WASHINGTON, USA," The Compass: Earth Science Journal of Sigma Gamma Epsilon: Vol. 89: Iss. 2, Article 1. Available at: https://digitalcommons.csbsju.edu/compass/vol89/iss2/1 This Article is brought to you for free and open access by DigitalCommons@CSB/SJU. It has been accepted for inclusion in The Compass: Earth Science Journal of Sigma Gamma Epsilon by an authorized editor of DigitalCommons@CSB/SJU. For more information, please contact [email protected]. SIGMA GAMMA EPSILON STUDENT RESEARCH POSTER SESSION, GEOLOGICAL SOCIETY OF AMERICA MEETING 2017, SEATTLE, WASHINGTON, USA Paula Even Sigma Gamma Epsilon Dept. of Earth Science University of Northern Iowa Cedar Falls, IA 50614-0335 USA [email protected] ABSTRACT The Sigma Gamma Epsilon, an academic honor society for students of the Earth Sciences, is an important tradition at GSA annual meetings. Sigma Gamma Epsilon's goal in sponsoring this session is to provide a student-friendly forum for young researchers to present on undergraduate research; this session has seen increasing interest and participation over the years. The session is open to students (regardless of membership in Sigma Gamma Epsilon) and faculty co-authors working in any area of the geosciences. -

Evolution of the Human Pelvis
COMMENTARY THE ANATOMICAL RECORD 300:789–797 (2017) Evolution of the Human Pelvis 1 2 KAREN R. ROSENBERG * AND JEREMY M. DESILVA 1Department of Anthropology, University of Delaware, Newark, Delaware 2Department of Anthropology, Dartmouth College, Hanover, New Hampshire ABSTRACT No bone in the human postcranial skeleton differs more dramatically from its match in an ape skeleton than the pelvis. Humans have evolved a specialized pelvis, well-adapted for the rigors of bipedal locomotion. Pre- cisely how this happened has been the subject of great interest and con- tention in the paleoanthropological literature. In part, this is because of the fragility of the pelvis and its resulting rarity in the human fossil record. However, new discoveries from Miocene hominoids and Plio- Pleistocene hominins have reenergized debates about human pelvic evolu- tion and shed new light on the competing roles of bipedal locomotion and obstetrics in shaping pelvic anatomy. In this issue, 13 papers address the evolution of the human pelvis. Here, we summarize these new contribu- tions to our understanding of pelvic evolution, and share our own thoughts on the progress the field has made, and the questions that still remain. Anat Rec, 300:789–797, 2017. VC 2017 Wiley Periodicals, Inc. Key words: pelvic evolution; hominin; Australopithecus; bipedalism; obstetrics When Jeffrey Laitman contacted us about coediting a (2017, this issue) finds that humans, like other homi- special issue for the Anatomical Record on the evolution noids, have high sacral variability with a large percent- of the human pelvis, we were thrilled. The pelvis is hot age of individuals possessing the non-modal number of right now—thanks to new fossils (e.g., Morgan et al., sacral vertebrae.