Anomalies in the Vertebral Column and Ilio-Sacral Articulation of Some Anuran Amphibians
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vertebral Column and Thorax
Introduction to Human Osteology Chapter 4: Vertebral Column and Thorax Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 The Vertebral Column and Thorax Sternum Manubrium – bone that is trapezoidal in shape, makes up the superior aspect of the sternum. Jugular notch – concave notches on either side of the superior aspect of the manubrium, for articulation with the clavicles. Corpus or body – flat, rectangular bone making up the major portion of the sternum. The lateral aspects contain the notches for the true ribs, called the costal notches. Xiphoid process – variably shaped bone found at the inferior aspect of the corpus. Process may fuse late in life to the corpus. Clavicle Sternal end – rounded end, articulates with manubrium. Acromial end – flat end, articulates with scapula. Conoid tuberosity – muscle attachment located on the inferior aspect of the shaft, pointing posteriorly. Ribs Scapulae Head Ventral surface Neck Dorsal surface Tubercle Spine Shaft Coracoid process Costal groove Acromion Glenoid fossa Axillary margin Medial angle Vertebral margin Manubrium. Left anterior aspect, right posterior aspect. Sternum and Xyphoid Process. Left anterior aspect, right posterior aspect. Clavicle. Left side. Top superior and bottom inferior. First Rib. Left superior and right inferior. Second Rib. Left inferior and right superior. Typical Rib. Left inferior and right superior. Eleventh Rib. Left posterior view and left superior view. Twelfth Rib. Top shows anterior view and bottom shows posterior view. Scapula. Left side. Top anterior and bottom posterior. Scapula. Top lateral and bottom superior. Clavicle Sternum Scapula Ribs Vertebrae Body - Development of the vertebrae can be used in aging of individuals. -

Amphibian Ark Conservation Needs Assessment, Philippines, July 2014 Page 1
Amphibian Ark Conservation Needs Assessment, Philippines, July 2014 Page 1 Species suited to Conservation Education 42 species Species that are specifically selected for management – primarily in zoos and aquariums - to inspire and increase knowledge in visitors, in order to promote positive behavioural change. For example, when a species is used to raise financial or other support for field conservation projects (this would include clearly defined ‘flagship’ or ‘ambassador’ species). Phylogenetic Cultural/socio-economic Scientific Education Species Biological Distinctiveness significance importance Importance potential Sanguirana everetti 8.659490771 No aspect of biology known to be No No research dependent on this Yes exceptional species Research into availability of founders needs to be prioritised. Known in the area it was first collected like Mt. Apo. Threat: Habitat loss. It is not seen in great numbers anymore in Mindanao and chytrid may already affected them – hard hit (Diesmos). New genetic data suggest that real S. everetti is a SW Mindanao species...not sure what the Apo population would be since it has not be included in a phylogenetic study (Brown). No inferences can be made on the basis of habitat or forest cover. Need actual surveys of populations (Brown). Recommended to be listed as Data Deficient, for its distribution. Data Deficient because no studies can be conducted in that region due to security issues (Brown). Hylarana similis 7.692704126 No aspect of biology known to be No No research dependent on this Yes exceptional species Chytrid infected, effects cannot be reversed in time, high priority, but (Diesmos) they are quiet common on other mountain areas in Luzon, one of the hardest hit frog. -

Maritime Southeast Asia and Oceania Regional Focus
November 2011 Vol. 99 www.amphibians.orgFrogLogNews from the herpetological community Regional Focus Maritime Southeast Asia and Oceania INSIDE News from the ASG Regional Updates Global Focus Recent Publications General Announcements And More..... Spotted Treefrog Nyctixalus pictus. Photo: Leong Tzi Ming New The 2012 Sabin Members’ Award for Amphibian Conservation is now Bulletin open for nomination Board FrogLog Vol. 99 | November 2011 | 1 Follow the ASG on facebook www.facebook.com/amphibiansdotor2 | FrogLog Vol. 99| November 2011 g $PSKLELDQ$UN FDOHQGDUVDUHQRZDYDLODEOH 7KHWZHOYHVSHFWDFXODUZLQQLQJSKRWRVIURP $PSKLELDQ$UN¶VLQWHUQDWLRQDODPSKLELDQ SKRWRJUDSK\FRPSHWLWLRQKDYHEHHQLQFOXGHGLQ $PSKLELDQ$UN¶VEHDXWLIXOZDOOFDOHQGDU7KH FDOHQGDUVDUHQRZDYDLODEOHIRUVDOHDQGSURFHHGV DPSKLELDQDUN IURPVDOHVZLOOJRWRZDUGVVDYLQJWKUHDWHQHG :DOOFDOHQGDU DPSKLELDQVSHFLHV 3ULFLQJIRUFDOHQGDUVYDULHVGHSHQGLQJRQ WKHQXPEHURIFDOHQGDUVRUGHUHG±WKHPRUH \RXRUGHUWKHPRUH\RXVDYH2UGHUVRI FDOHQGDUVDUHSULFHGDW86HDFKRUGHUV RIEHWZHHQFDOHQGDUVGURSWKHSULFHWR 86HDFKDQGRUGHUVRIDUHSULFHGDW MXVW86HDFK 7KHVHSULFHVGRQRWLQFOXGH VKLSSLQJ $VZHOODVRUGHULQJFDOHQGDUVIRU\RXUVHOIIULHQGV DQGIDPLO\ZK\QRWSXUFKDVHVRPHFDOHQGDUV IRUUHVDOHWKURXJK\RXU UHWDLORXWOHWVRUIRUJLIWV IRUVWDIIVSRQVRUVRUIRU IXQGUDLVLQJHYHQWV" 2UGHU\RXUFDOHQGDUVIURPRXUZHEVLWH ZZZDPSKLELDQDUNRUJFDOHQGDURUGHUIRUP 5HPHPEHU±DVZHOODVKDYLQJDVSHFWDFXODUFDOHQGDU WRNHHSWUDFNRIDOO\RXULPSRUWDQWGDWHV\RX¶OODOVREH GLUHFWO\KHOSLQJWRVDYHDPSKLELDQVDVDOOSUR¿WVZLOOEH XVHGWRVXSSRUWDPSKLELDQFRQVHUYDWLRQSURMHFWV ZZZDPSKLELDQDUNRUJ FrogLog Vol. 99 | November -

Skeletal System? Skeletal System Chapters 6 & 7 Skeletal System = Bones, Joints, Cartilages, Ligaments
Warm-Up Activity • Fill in the names of the bones in the skeleton diagram. Warm-Up 1. What are the 4 types of bones? Give an example of each. 2. Give 3 ways you can tell a female skeleton from a male skeleton. 3. What hormones are involved in the skeletal system? Skeletal System Chapters 6 & 7 Skeletal System = bones, joints, cartilages, ligaments • Axial skeleton: long axis (skull, vertebral column, rib cage) • Appendicular skeleton: limbs and girdles Appendicular Axial Skeleton Skeleton • Cranium (skull) • Clavicle (collarbone) • Mandible (jaw) • Scapula (shoulder blade) • Vertebral column (spine) • Coxal (pelvic girdle) ▫ Cervical vertebrae • Humerus (arm) ▫ Thoracic vertebrae • Radius, ulna (forearm) ▫ Lumbar vertebrae • Carpals (wrist) • Metacarpals (hand) ▫ Sacrum • Phalanges (fingers, toes) ▫ Coccyx • Femur (thigh) • Sternum (breastbone) • Tibia, fibula (leg) • Ribs • Tarsal, metatarsals (foot) • Calcaneus (heel) • Patella (knee) Functions of the Bones • Support body and cradle soft organs • Protect vital organs • Movement: muscles move bones • Storage of minerals (calcium, phosphorus) & growth factors • Blood cell formation in bone marrow • Triglyceride (fat) storage Classification of Bones 1. Long bones ▫ Longer than they are wide (eg. femur, metacarpels) 2. Short bones ▫ Cube-shaped bones (eg. wrist and ankle) ▫ Sesamoid bones (within tendons – eg. patella) 3. Flat bones ▫ Thin, flat, slightly curved (eg. sternum, skull) 4. Irregular bones ▫ Complicated shapes (eg. vertebrae, hips) Figure 6.2 • Adult = 206 bones • Types of bone -

Early Tetrapod Relationships Revisited
Biol. Rev. (2003), 78, pp. 251–345. f Cambridge Philosophical Society 251 DOI: 10.1017/S1464793102006103 Printed in the United Kingdom Early tetrapod relationships revisited MARCELLO RUTA1*, MICHAEL I. COATES1 and DONALD L. J. QUICKE2 1 The Department of Organismal Biology and Anatomy, The University of Chicago, 1027 East 57th Street, Chicago, IL 60637-1508, USA ([email protected]; [email protected]) 2 Department of Biology, Imperial College at Silwood Park, Ascot, Berkshire SL57PY, UK and Department of Entomology, The Natural History Museum, Cromwell Road, London SW75BD, UK ([email protected]) (Received 29 November 2001; revised 28 August 2002; accepted 2 September 2002) ABSTRACT In an attempt to investigate differences between the most widely discussed hypotheses of early tetrapod relation- ships, we assembled a new data matrix including 90 taxa coded for 319 cranial and postcranial characters. We have incorporated, where possible, original observations of numerous taxa spread throughout the major tetrapod clades. A stem-based (total-group) definition of Tetrapoda is preferred over apomorphy- and node-based (crown-group) definitions. This definition is operational, since it is based on a formal character analysis. A PAUP* search using a recently implemented version of the parsimony ratchet method yields 64 shortest trees. Differ- ences between these trees concern: (1) the internal relationships of aı¨stopods, the three selected species of which form a trichotomy; (2) the internal relationships of embolomeres, with Archeria -
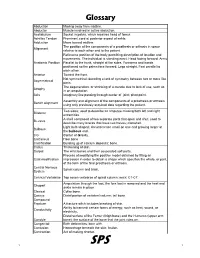
Glossary of Basic Orthotic & Prosthetic Terminology
Glossary Abduction Moving away from midline. Abductor Muscle involved in active abduction. Acetabulum Socket in pelvis, which receives head of femur. Achilles Tendon Prominent cord at posterior aspect of ankle. Adduction Move toward midline. The position of the components of a prosthesis or orthosis in space Alignment relative to each other and to the patient. Reference position of the body permitting description of location and movements. The individual is standing erect. Head facing forward. Arms Anatomic Position Parallel to the trunk, straight at the sides. Forearms and hands positioned so the palms face forward. Legs straight. Feet parallel to each other. Anterior Toward the front. Not symmetrical; denoting a lack of symmetry between two or more like Asymmetrical parts. The degeneration, or shrinking of a muscle due to lack of use, such as Atrophy in an amputation. Axis Imaginary line passing through center of joint; pivot point. Assembly and alignment of the components of a prosthesis or orthosis Bench alignment using only previously acquired data regarding the patient. Two sides; used to describe an amputee missing both left and right Bilateral extremities. A shell composed of two separate parts that open and shut; used to Bi-valve describe many braces that have two halves; clamshell. Light bulb shaped; circumference small on one end growing larger at Bulbous the bulbous end. CG Center of Gravity. Calcaneus Heel bone Calcification Building up of calcium deposits; bone. Callus Thickening of skin. Carpal The wrist bones and their associated soft parts. Process of modifying the positive model obtained by filling an Cast modification impression in order to obtain a shape which specifies the whole, or part, of the form of the final prosthesis or orthosis. -

Vertebral Column
Vertebral Column • Backbone consists of Cervical 26 vertebrae. • Five vertebral regions – Cervical vertebrae (7) Thoracic in the neck. – Thoracic vertebrae (12) in the thorax. – Lumbar vertebrae (5) in the lower back. Lumbar – Sacrum (5, fused). – Coccyx (4, fused). Sacrum Coccyx Scoliosis Lordosis Kyphosis Atlas (C1) Posterior tubercle Vertebral foramen Tubercle for transverse ligament Superior articular facet Transverse Transverse process foramen Facet for dens Anterior tubercle • Atlas- ring of bone, superior facets for occipital condyles. – Nodding movement signifies “yes”. Axis (C2) Spinous process Lamina Vertebral foramen Transverse foramen Transverse process Superior articular facet Odontoid process (dens) •Axis- dens or odontoid process is body of atlas. – Pivotal movement signifies “no”. Typical Cervical Vertebra (C3-C7) • Smaller bodies • Larger spinal canal • Transverse processes –Shorter – Transverse foramen for vertebral artery • Spinous processes of C2 to C6 often bifid • 1st and 2nd cervical vertebrae are unique – Atlas & axis Typical Cervical Vertebra Spinous process (bifid) Lamina Vertebral foramen Inferior articular process Superior articular process Transverse foramen Pedicle Transverse process Body Thoracic Vertebrae (T1-T12) • Larger and stronger bodies • Longer transverse & spinous processes • Demifacets on body for head of rib • Facets on transverse processes (T1-T10) for tubercle of rib Thoracic Vertebra- superior view Spinous process Transverse process Facet for tubercle of rib Lamina Superior articular process -

OP-MOLB160269 Online 744..771
Evolutionary History of the Asian Horned Frogs (Megophryinae): Integrative Approaches to Timetree Dating in the Absence of a Fossil Record Stephen Mahony,*,1,2 Nicole M. Foley,1 S.D. Biju,2 and Emma C. Teeling*,1 1School of Biology and Environmental Science, University College Dublin, Belfield, Dublin, Ireland 2Systematics Lab, Department of Environmental Studies, University of Delhi, Delhi, India *Corresponding authors: E-mails: [email protected]; [email protected]. Associate editor: Beth Shapiro Abstract Downloaded from https://academic.oup.com/mbe/article-abstract/34/3/744/2919384 by guest on 06 August 2019 Molecular dating studies typically need fossils to calibrate the analyses. Unfortunately, the fossil record is extremely poor or presently nonexistent for many species groups, rendering such dating analysis difficult. One such group is the Asian horned frogs (Megophryinae). Sampling all generic nomina, we combined a novel 5 kb dataset composed of four nuclear and three mitochondrial gene fragments to produce a robust phylogeny, with an extensive external morpho- logical study to produce a working taxonomy for the group. Expanding the molecular dataset to include out-groups of fossil-represented ancestral anuran families, we compared the priorless RelTime dating method with the widely used prior-based Bayesian timetree method, MCMCtree, utilizing a novel combination of fossil priors for anuran phylogenetic dating. The phylogeny was then subjected to ancestral phylogeographic analyses, and dating estimates were compared with likely biogeographic vicariant events. Phylogenetic analyses demonstrated that previously proposed systematic hypotheses were incorrect due to the paraphyly of genera. Molecular phylogenetic, morphological, and timetree results support the recognition of Megophryinae as a single genus, Megophrys, with a subgenus level classification. -

Philippine Amphibians and Reptiles: an Overview of Species Diversity, Biogeography, and Conservation
1 Philippine Amphibians and Reptiles: An Overview of Species Diversity, Biogeography, and Conservation Arvin C. Diesmos, Rafe M. Brown , Angel C. Alcala!, Rogelio V. Sison", Leticia E. Afuang#, and Genevieve V. A. Gee$ Biological Sciences Department, College of Science, De La Salle University-Dasmariñas, 4115 Dasmariñas, Cavite, Philippines; Section of Integrative Biology and Texas Memorial Museum, University of Texas, Austin, TX 78712, USA; !Silliman University- Angelo King Center for Research and Environmental Management, SU Marine Laboratory, 6200 Dumaguete City, Philippines; "Herpetology Section, Zoology Division, Philippine National Museum, Padre Burgos Street, 1000 Manila, Philippines; #Conservation International-Philippines, Philam Homes, 1104 Quezon City, Philippines; $Haribon Foundation, Teachers Village, 1101 Quezon City, Philippines 6he Philippine Archipelago is home to a spec- flat-headed frog (Barbourula busuangensis), a fully tacular and diverse assemblage of amphibians and rep- aquatic species inhabiting unpolluted mountain streams tiles. Situated at the interface between the Oriental and and rivers of Palawan. It is one of the only two known Australian faunal zones, the herpetofauna of this largely species of this genus. Frogs of the genus Platymantis oceanic island archipelago has captured the attention are the most diverse; the number of Philippine species and imagination of systematists and biogeographers for currently stands at 26 but dozens of newly discovered nearly 200 years. Previously thought of as having a species are still awaiting description. depauperate herpetofauna, the Philippine archipelago is now recognized as one of the most important center The fauna includes four species of frogs that have of herpetofaunal diversity in Southeast Asia. Informa- been introduced by man either intentionally (Bufo tion generated from recent field surveys coupled with marinus, Hoplobatrachus rugulosus, Rana catesbeiana) analyses of available data on diversity, points to over- or inadvertently (Rana erythraea). -

Cervical Vertebrae 1 Cervical Vertebrae
Cervical vertebrae 1 Cervical vertebrae Cervical vertebrae or Cervilar Position of human cervical vertebrae (shown in red). It consists of 7 bones, from top to bottom, C1, C2, C3, C4, C5, C6 and C7. A human cervical vertebra Latin Vertebrae cervicales [1] Gray's p.97 [2] MeSH Cervical+vertebrae [3] TA A02.2.02.001 [4] FMA FMA:72063 In vertebrates, cervical vertebrae (singular: vertebra) are those vertebrae immediately inferior to the skull. Thoracic vertebrae in all mammalian species are defined as those vertebrae that also carry a pair of ribs, and lie caudal to the cervical vertebrae. Further caudally follow the lumbar vertebrae, which also belong to the trunk, but do not carry ribs. In reptiles, all trunk vertebrae carry ribs and are called dorsal vertebrae. In many species, though not in mammals, the cervical vertebrae bear ribs. In many other groups, such as lizards and saurischian dinosaurs, the cervical ribs are large; in birds, they are small and completely fused to the vertebrae. The transverse processes of mammals are homologous to the cervical ribs of other amniotes. Cervical vertebrae 2 In humans, cervical vertebrae are the smallest of the true vertebrae, and can be readily distinguished from those of the thoracic or lumbar regions by the presence of a foramen (hole) in each transverse process, through which passes the vertebral artery. The remainder of this article focuses upon human anatomy. Structure By convention, the cervical vertebrae are numbered, with the first one (C1) located closest to the skull and higher numbered vertebrae (C2-C7) proceeding away from the skull and down the spine. -

The Conservation Status of Biological Resources in the Philippines
: -.^,rhr:"-i-3'^^=£#?^-j^.r-^a^ Sj2 r:iw0,">::^^'^ \^^' Cfl|*ti-»;;^ THE CONSERVATION STATUS OF BIOLOGICAL RESOURCES IN THE PHILIPPINES A RRF'OHT V^Y THK lUCN CONSKRVATION MONITORING CENT:-!E PfcparGd by Roger Cox for the lnLf5rnaLion?.l InsLituLo Cor Knvironment and Development (IIED) February 1988 / fgrMsa^jnt-^'-agyga-- •r-r- ;.«-'> t ^-' isr* 1*.- i^^s. , r^^, ^».|;; ^b-^ ^.*%-^ *i,r^-v . iinnc [ '»/' C'A'. aSM!': Vi - '«.;s^ ; a-* f%h '3;riti7;.:- n'^'ji K ;ii;!'r ' <s:ii.uiy.. viii. K A xo.^ jf^'r;.' 3 10 ciJuJi i\ Ji\{ :::) Jnj:kf- .i. n ( im'.i) •V'lt r'v - -V.-^f~^?fl LP-ife- f^^ s.:.... --11 -^M.jj^^^ riB CC./Sfc^RvAriON .<*TC.rj^. OF EI3U:i' "I.VJ, JbO'TSOURCES ^^a THE PHILIPPlVl'fC ;j^...^..-r'^^ I ilRPOHT BY THK ILCJJ CGJJSIiKVA'ilCN M0N:.V:..):;1NG CKNT ^ Pc'jpas-fjr' ')y Roto* C(/X for the TiKD). {'obruary 1988 Digitized by the Internet Archive in 2010 with funding from UNEP-WCIVIC, Cambridge http://www.archive.org/details/conservationstat88coxr . 7' CONTENTS List of Figures, Appendices and Tables iii Summary iy Acknowledgements vii 1 INTRODUCTION 1.1 Background 1 1.2 Objectives 3 2 METHODS 4 3. FLORA, VEGETATION AND FOREST COVER 3.1 Description of the natural vegetation 4 3.1.1 The forests 4 3.1.2 Other vegetation types 7 3 2 Conservation status of the Philippine flora 8 3.2.1 Introduction 8 3.2.2 Causes of habitat destruction 9 3.2.3 Threatened plant species 11 3. 2. A Centres of plant diversity and endemism 12 4 COASTAL AND MARINE ECOSYSTEMS 4.1 Background 17 4.2 Mangroves 18 4.3 Coral reefs 19 4.4 Seagrass beds 22 5. -

Clinical Anatomy of the Female Pelvis 1
Clinical Anatomy of the Female Pelvis 1 Clinical Anatomy of the Female Pelvis 1 Helga Fritsch CONTENTS 1.1 Introduction 1.1 Introduction 1 1.2 Morphological and The pelvic fl oor constitutes the caudal border of the Clinical Subdivision of the Female Pelvis 1 human’s visceral cavity. It is characterized by a com- plex morphology because different functional systems 1.3 Compartments 7 join here. A clear understanding of the pelvic anatomy 1.3.1 Posterior Compartment 7 1.3.1.1 Connective Tissue Structures 7 is crucial for the diagnosis of female pelvic diseases, for 1.3.1.2 Muscles 10 female pelvic surgery as well as for fundamental mech- 1.3.1.3 Reinterpreted Anatomy and anisms of urogenital dysfunction and treatment. Clinical Relevance 12 Modern imaging techniques are used for the diag- 1.3.1.4 Important Vessels, Nerves and Lymphatics of the Posterior Compartment: 13 nosis of pelvic fl oor or sphincter disorders. Further- 1.3.2 Anterior Compartment 14 more, they are employed to determine the extent of 1.3.2.1 Connective Tissue Structures 14 pelvic diseases and the staging of pelvic tumors. In 1.3.2.2 Muscles 15 order to be able to recognize the structures seen on 1.3.2.3 Reinterpreted Anatomy and CT and MRI as well as on dynamic MRI, a detailed Clinical Relevance 16 1.3.2.4 Important Vessels, Nerves and Lymphatics knowledge of the relationship of the anatomical enti- of the Anterior Compartment: 16 ties within the pelvic anatomy is required. 1.3.3 Middle Compartment 17 The Terminologia Anatomica [15] contains a mix- 1.3.3.1 Connective Tissue Structures 17 ture of old and new terms describing the different 1.3.3.2 Muscles 17 structures of the pelvis.