Roco Proteins and the Parkinson's Disease-Associated LRRK2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulation of Dishevelled DEP Domain Swapping by Conserved Phosphorylation Sites
Regulation of Dishevelled DEP domain swapping by conserved phosphorylation sites Gonzalo J. Beitiaa,1, Trevor J. Rutherforda,1, Stefan M. V. Freunda, Hugh R. Pelhama, Mariann Bienza, and Melissa V. Gammonsa,2 aMedical Research Council Laboratory of Molecular Biology, Cambridge Biomedical Campus, Cambridge, CB2 0QH, United Kingdom Edited by Roeland Nusse, Stanford University School of Medicine, Stanford, CA, and approved May 13, 2021 (received for review February 20, 2021) Wnt signals bind to Frizzled receptors to trigger canonical and with these effectors even if present at a low cellular concentra- noncanonical signaling responses that control cell fates during tion (1, 3, 12). animal development and tissue homeostasis. All Wnt signals are The DEP domain is a small globular domain composed of three relayed by the hub protein Dishevelled. During canonical (β-catenin– α-helices and a flexible hinge loop between the first (H1) and sec- dependent) signaling, Dishevelled assembles signalosomes via dy- ond helix (H2), which, in the monomeric configuration, folds back namic head-to-tail polymerization of its Dishevelled and Axin (DIX) on itself to form a prominent “DEP finger” that is responsible domain, which are cross-linked by its Dishevelled, Egl-10, and Pleck- for binding to Frizzled (Fig. 1A) (13). DEP dimerization involves strin (DEP) domain through a conformational switch from monomer a highly unusual mechanism called “domain swapping” (14). During to domain-swapped dimer. The domain-swapped conformation of this process, H1 of one DEP monomer is exchanged with H1 from a DEP masks the site through which Dishevelled binds to Frizzled, im- reciprocal one through outward motions of the hinge loops, replacing plying that DEP domain swapping results in the detachment of Dish- intra- with intermolecular contacts. -

PDZ Domain from Dishevelled — a Specificity Study Katarzyna Śmietana, Agnieszka Mateja, Artur Krężel and Jacek Otlewski*
Vol. 58, No. 2/2011 243–249 on-line at: www.actabp.pl Regular paper PDZ domain from Dishevelled — a specificity study Katarzyna Śmietana, Agnieszka Mateja, Artur Krężel and Jacek Otlewski* Faculty of Biotechnology, Department of Protein Engineering, University of Wrocław, Wrocław, Poland Intracellular signaling cascades induced by Wnt proteins play a key role in developmental processes and are im- It is still unclear what mechanism governs the activa- plicated in cancerogenesis. It is still unclear how the cell tion of the correct Wnt signaling branch. Dishevelled determines which of the three possible Wnt response (Dvl) proteins, the last shared link of the canonical and mechanisms should be activated, but the decision proc- non-canonical pathways, most likely play a key regulatory ess is most likely dependent on Dishevelled proteins. role in the signal distribution process (Habas & Dawid, Dishevelled family members interact with many diverse 2005; Itoh et al., 2005; Leonard & Ettensohn, 2007). This targets, however, molecular mechanisms underlying notion is further supported by the fact that the multi- these binding events have not been comprehensively tude of Dvl binding partners includes proteins that re- described so far. Here, we investigated the specificity of lay signaling to β-catenin pathways (axin (Li et al., 1999; the PDZ domain from human Dishevelled-2 using C-ter- Wharton, 2003), GBP/Frat (Li et al., 1999)), PCP (Rac1 minal phage display, which led us to identification of a (Fanto et al., 2000), Daam1 (Habas et al., 2001), strabis- 2+ leucine-rich binding motif strongly resembling the con- mus (Bastock et al., 2003)) and Ca (Gαo/Gαt (Liu et al., sensus sequence of a nuclear export signal. -

Supp Table 6.Pdf
Supplementary Table 6. Processes associated to the 2037 SCL candidate target genes ID Symbol Entrez Gene Name Process NM_178114 AMIGO2 adhesion molecule with Ig-like domain 2 adhesion NM_033474 ARVCF armadillo repeat gene deletes in velocardiofacial syndrome adhesion NM_027060 BTBD9 BTB (POZ) domain containing 9 adhesion NM_001039149 CD226 CD226 molecule adhesion NM_010581 CD47 CD47 molecule adhesion NM_023370 CDH23 cadherin-like 23 adhesion NM_207298 CERCAM cerebral endothelial cell adhesion molecule adhesion NM_021719 CLDN15 claudin 15 adhesion NM_009902 CLDN3 claudin 3 adhesion NM_008779 CNTN3 contactin 3 (plasmacytoma associated) adhesion NM_015734 COL5A1 collagen, type V, alpha 1 adhesion NM_007803 CTTN cortactin adhesion NM_009142 CX3CL1 chemokine (C-X3-C motif) ligand 1 adhesion NM_031174 DSCAM Down syndrome cell adhesion molecule adhesion NM_145158 EMILIN2 elastin microfibril interfacer 2 adhesion NM_001081286 FAT1 FAT tumor suppressor homolog 1 (Drosophila) adhesion NM_001080814 FAT3 FAT tumor suppressor homolog 3 (Drosophila) adhesion NM_153795 FERMT3 fermitin family homolog 3 (Drosophila) adhesion NM_010494 ICAM2 intercellular adhesion molecule 2 adhesion NM_023892 ICAM4 (includes EG:3386) intercellular adhesion molecule 4 (Landsteiner-Wiener blood group)adhesion NM_001001979 MEGF10 multiple EGF-like-domains 10 adhesion NM_172522 MEGF11 multiple EGF-like-domains 11 adhesion NM_010739 MUC13 mucin 13, cell surface associated adhesion NM_013610 NINJ1 ninjurin 1 adhesion NM_016718 NINJ2 ninjurin 2 adhesion NM_172932 NLGN3 neuroligin -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

DEP Domains: Ner in Vivo
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Developmental Cell 436 important proteins whose functions depend on post- Jechlinger, M., Grunert, S., Tamir, I.H., Janda, E., Ludemann, S., transcriptional control are being identified, this study Waerner, T., Seither, P., Weith, A., Beug, H., and Kraut, N. (2003). provides a further motivation to apply tools for gene Oncogene 22, 7155–7169. identification that take these modes of regulation into Muller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M.E., McClanahan, T., Murphy, E., Yuan, W., Wagner, S.N., et al. (2001). consideration. Nature 410, 50–56. 1 1 Orimo, A., Gupta, P.B., Sgroi, D.C., Arenzana-Seisdedos, F., Delau- Natalia C. Mackenzie and Erez Raz nay, T., Naeem, R., Carey, V.J., Richardson, A.L., and Weinberg, 1 Germ Cell Development R.A. (2005). Cell 121, 335–348. Max-Planck-Institute for Biophysical Chemistry Pradet-Balade, B., Boulme, F., Beug, H., Mullner, E.W., and Garcia- Am Fassberg 11 Sanz, J.A. (2001). Trends Biochem. Sci. 26, 225–229. Go¨ ttingen 37070 Shook, D., and Keller, R. (2003). Mech. Dev. 120, 1351–1383. Germany Thiery, J.P., and Sleeman, J.P. (2006). Nat. Rev. Mol. Cell Biol. 7, 131–142. Selected Reading Waerner, T., Alacakaptan, M., Tamir, I., Oberauer, R., Gal, A., Bra- bletz, T., Schreiber, M., Jechlinger, M., and Beug, H. (2006). Cancer Castanon, I., and Baylies, M.K. (2002). Gene 287, 11–22. Cell 10, 227–239. Janda, E., Lehmann, K., Killisch, I., Jechlinger, M., Herzig, M., Yang, J., Mani, S.A., Donaher, J.L., Ramaswamy, S., Itzykson, R.A., Downward, J., Beug, H., and Grunert, S. -
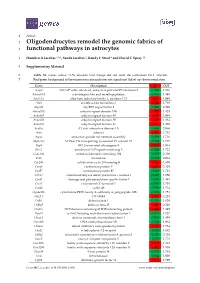
Oligodendrocytes Remodel the Genomic Fabrics of Functional Pathways in Astrocytes
1 Article 2 Oligodendrocytes remodel the genomic fabrics of 3 functional pathways in astrocytes 4 Dumitru A Iacobas 1,2,*, Sanda Iacobas 3, Randy F Stout 4 and David C Spray 2,5 5 Supplementary Material 6 Table S1. Genes whose >1.5x absolute fold-change did not meet the individual CUT criterion. 7 Red/green background of the expression ratio indicates not significant (false) up-/down-regulation. Gene Description X CUT Acap2 ArfGAP with coiled-coil, ankyrin repeat and PH domains 2 -1.540 1.816 Adamts18 a disintegrin-like and metallopeptidase -1.514 1.594 Akr1c12 aldo-keto reductase family 1, member C12 1.866 1.994 Alx3 aristaless-like homeobox 3 1.536 1.769 Alyref2 Aly/REF export factor 2 -1.880 2.208 Ankrd33b ankyrin repeat domain 33B 1.593 1.829 Ankrd45 ankyrin repeat domain 45 1.514 1.984 Ankrd50 ankyrin repeat domain 50 1.628 1.832 Ankrd61 ankyrin repeat domain 61 1.645 1.802 Arid1a AT rich interactive domain 1A -1.668 2.066 Artn artemin 1.524 1.732 Aspm abnormal spindle microtubule assembly -1.693 1.716 Atp6v1e1 ATPase, H+ transporting, lysosomal V1 subunit E1 -1.679 1.777 Bag4 BCL2-associated athanogene 4 1.723 1.914 Birc3 baculoviral IAP repeat-containing 3 -1.588 1.722 Ccdc104 coiled-coil domain containing 104 -1.819 2.130 Ccl2 chemokine -1.699 2.034 Cdc20b cell division cycle 20 homolog B 1.512 1.605 Cenpf centromere protein F 2.041 2.128 Cep97 centrosomal protein 97 -1.641 1.723 COX1 mitochondrially encoded cytochrome c oxidase I -1.607 1.650 Cpsf7 cleavage and polyadenylation specific factor 7 -1.635 1.891 Crct1 cysteine-rich -

Involvement of Upregulation of DEPDC1 (DEP Domain Containing 1) in Bladder Carcinogenesis
Oncogene (2007) 26, 6448–6455 & 2007 Nature Publishing Group All rights reserved 0950-9232/07 $30.00 www.nature.com/onc SHORT COMMUNICATION Involvement of upregulation of DEPDC1 (DEP domain containing 1) in bladder carcinogenesis M Kanehira1,2,5, Y Harada1,5, R Takata1,2, T Shuin3, T Miki4, T Fujioka2, Y Nakamura1 and T Katagiri1 1Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo, Minato-ku, Tokyo, Japan; 2Department of Urology, Iwate Medical University, Morioka, Japan; 3Department of Urology, Kochi Medical School, Nankoku, Japan and 4Department of Urology, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto, Japan In an attempt to disclose mechanisms of bladder toward understanding of the factors involved in the carcinogenesis and discover novel target molecules for progression of bladder cancer will facilitate develop- development of treatment, we applied a cDNA microarray ment of molecular-based diagnostic and therapeutic to screen genes that were significantly transactivated in approaches. In the last two decades, cisplatin-based bladder cancer cells. Among the upregulated genes, we combination chemotherapy regimens, such as CMV here focused on a novel gene, (DEPDC1) DEP domain (cisplatin, methotrexate and vinblastine) or M-VAC containing 1, whose overexpression was confirmed by (methotrexate, vinblastine, doxorubicin and cisplatin) northern blot and immunohistochemical analyses. Immu- have been mainly applied to patients with advanced nocytochemical staining analysis detected strong staining bladder cancers (Lehmann et al., 2002; Ardavanis et al., of endogenous DEPDC1protein in the nucleus of bladder 2005; Rosenberg et al., 2005; Theodore et al., 2005). cancer cells. Since DEPDC1 expression was hardly However, the overall prognosis remains still very poor detectable in any of 24 normal human tissues we examined and adverse reactions caused by these combination except the testis, we considered this gene-product to be a chemotherapies are significantly severe (Vaughn, 1999). -

Wnt/Β-Catenin Signaling Requires Interaction of the Dishevelled DEP
Wnt/β-catenin signaling requires interaction of the PNAS PLUS Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled Daniele V. F. Taurielloa,1,2, Ingrid Jordensa,1, Katharina Kirchnerb,3, Jerry W. Slootstrac,3, Tom Kruitwagena, Britta A. M. Bouwmana, Maria Noutsoua, Stefan G. D. Rüdigerd, Klaus Schwambornc, Alexandra Schambonyb, and Madelon M. Mauricea,4 aDepartment of Cell Biology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; bDevelopmental Biology Unit, Department of Biology and Developmental Biology, Friedrich-Alexander University Erlangen-Nuremberg, 91058 Erlangen, Germany; cPepscan Therapeutics BV, 8243 RC Lelystad, The Netherlands; and dCellular Protein Chemistry, Bijvoet Center for Biomolecular Research, Utrecht University, Utrecht, 3584 CH Utrecht, The Netherlands Edited by Roeland Nusse, Stanford University School of Medicine, Stanford, CA, and approved February 17, 2012 (received for review September 10, 2011) Wnt binding to members of the seven-span transmembrane croinjection of peptides comprising this motif interferes with Frizzled (Fz) receptor family controls essential cell fate decisions Wnt/β-catenin signaling in Xenopus embryos, further supporting and tissue polarity during development and in adulthood. The Fz- the functional importance of the Fz C-terminal tail. Mutagenesis mediated membrane recruitment of the cytoplasmic effector studies have revealed, however, that other Fz regions, including Dishevelled (Dvl) is a critical step in Wnt/β-catenin signaling initi- the intracellular loops, contribute to Wnt signaling by unknown ation, but how Fz and Dvl act together to drive downstream sig- mechanisms (14, 16–18). Indeed, overexpressed Drosophila Fz2 naling events remains largely undefined. Here, we use an Fz lacking its C-terminal tail still synergizes with Wnt in Dvl-de- peptide-based microarray to uncover a mechanistically important pendent signaling (19). -

Role of Molecular Chaperonin CCT and Its Co-Chaperone Phlp1 in the Assembly of Mtor Complexes
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2017-08-01 Role of Molecular Chaperonin CCT and Its Co-Chaperone PhLP1 in the Assembly of mTOR Complexes Madhura Vinayak Dhavale Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Chemistry Commons BYU ScholarsArchive Citation Dhavale, Madhura Vinayak, "Role of Molecular Chaperonin CCT and Its Co-Chaperone PhLP1 in the Assembly of mTOR Complexes" (2017). Theses and Dissertations. 6942. https://scholarsarchive.byu.edu/etd/6942 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Role of Molecular Chaperonin CCT and Its Co-Chaperone PhLP1 in the Assembly of mTOR Complexes Madhura Vinayak Dhavale A dissertation submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Barry M. Willardson, Chair Joshua L. Andersen Kenneth A. Christensen John C. Price Michael R. Stark Department of Chemistry and Biochemistry Brigham Young University Copyright © 2017 Madhura Vinayak Dhavale All Rights Reserved ABSTRACT Role of Molecular Chaperonin CCT and Its Co-Chaperone PhLP1 in the Assembly of mTOR Complexes Madhura Vinayak Dhavale Department of Chemistry and Biochemistry, BYU Doctor of Philosophy mTOR is the central kinase in biochemical pathways that regulate cellular growth, protein synthesis and cell survival. Deregulation of mTOR signaling results in uncontrolled cell proliferation and hence is implicated in various cancers and autoimmune diseases. -
Single Molecule Dynamics of Dishevelled at the Plasma Membrane and Wnt Pathway Activation
bioRxiv preprint doi: https://doi.org/10.1101/624882; this version posted May 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Classification: Biological Science, Systems Biology Single molecule dynamics of Dishevelled at the plasma membrane and Wnt pathway activation Wenzhe Maa*, Maorong Chenb*, Hong Kanga, Zachary Steinhartc, Stephane Angersc, Xi Heb,1, Marc W. Kirschnera,1 a Department of Systems Biology, Harvard Medical School, Boston, MA 02115, USA; b F. M. Kirby Neurobiology Center, Children's Hospital Boston, Harvard Medical School, Boston, MA 02115, USA c University of Toronto, 144 College Street, Toronto, ON M5S 3M2, Canada * The authors contribute equally to this work. For correspondence to: [email protected], [email protected] Keywords: Wnt Signaling Pathway, Disheveled, Single Molecule, Protein Complex Size, Fluorescence, TIRF (Total Intensity Reflection Fluorescence), Kinetic Models of complex assembly. Author contributions: W.M., M.C., X.H. and M.W.K. designed research; W.M., M.C. and H.K. performed research; Z.S. and S.A. contributed new reagents; W.M. and M.C. analyzed data; W.M. built mathematical models; W.M. and M.W.K wrote the paper. bioRxiv preprint doi: https://doi.org/10.1101/624882; this version posted May 8, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Dvl (Dishevelled) is one of several essential non-enzymatic components of the Wnt signaling pathway. -

Supplemental Figure 1
A BCD6 35 80 1.0 3 Thr Ala 0.4 Thr Ala 30 0.8 60 4 0.3 2 25 0.6 40 20 0.2 0.4 1 2 20 intake(g)Food Body weight(g) 15 0.1 0.2 Serum TSH (ng/ml) T4 plasmaT4 levels (ng/ml) plasma levelsT3 (ng/ml) 1/V 10 0.0 0 0.0 0 5 10 15 Thr Ala Thr Ala Thr Ala -1.0 -0.5 0.5 1.0 1.5 2.0 Thr Ala Thr Ala Age (weeks) 1/[T4] (nM) dark light (fmols/min/mg ptn) (fmols/min/mg -1 Ala Thr IKMN O Femur 16.5 0.55 1100 0.24 E F 16.0 1050 0.22 G H 0.50 Thr92‐D2 15.5 1000 0.20 Ala92‐D2 0.45 15.0 950 0.18 (mgHAcm-3) Femur volume 0.40 14.5 900 0.16 Cortical thickness Femur length (mm) length Femur Bone MineralDensity 14.0 0.35 850 0.14 Thr Ala Thr Ala Thr Ala Thr Ala JLPQR Vertebrae 4.6 5.5 0.070 0.35 4.4 5.0 0.065 0.30 **P<0.01 0.060 4.2 *** 4.5 0.055 0.25 4.0 4.0 0.050 0.20 3.8 3.5 Trabecular number 0.045 Trabecular spacing Trabecular thickness Vertebral height (mm) height Vertebral 3.6 3.0 0.040 0.15 Thr Ala Thr Ala Thr Ala Thr Ala Figure S1: Additional phenotype of the Ala92‐Dio2 mouse. -

DEP Domain Is Responsible for Membrane Translocation of Dishevelled-1
ARTICLE Cell Research (2004); 14(4):324-330 Role of DEP domain in membrane translocation of Dvl-1 http://www.cell-research.com http://www.cell-research.com Characterization of Function of Three Domains in Dishevelled-1: DEP Domain is Responsible for Membrane Translocation of Dishevelled-1 Wei Jun PAN1*, Shu Zhao PANG1,2*, Tao HUANG1, Hui Yun GUO2, Dianqing WU3, Lin LI1** 1Laboratory of Molecular Cell Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Graduate School of the Chinese Academy of Sciences, 320 Yueyang Rd, Shanghai 200031; 2College of Life Science, Jilin University, Changchun 130061, China. 3Department of Genetics and Developmental Biology, University of Connecticut Health Center, Farmington, Connecticut 06030, USA. ABSTRACT Wnt signaling plays an important role in embryogenesis and tumorgenesis. Although the mechanism about how Wnts transduce their signaling from receptor frizzled (Fz) to cytosol has not been understood, dishevelled (Dvl) protein was considered as the intersection of Wnt signal traffic. In this study, we characterized the function of three domains (DIX, PDZ and DEP) of Dvl-1 in canonical Wnt signal transduction and Dvl-1 membrane translocation. It was found both DIX and DEP domain were sufficient to block Wnt-3a-induced LEF-1 transcriptional activity and free cytosol β- catenin accumulation; whereas PDZ domain and a functional mutant form of DEP domain (DEP-KM) had no effect on canonical Wnt signaling. In addition, when cotransfected with Fz-7, DEP domain, but not DIX, PDZ or DEP-KM, translocated and co-localized with Fz-7 to the plasma membrane, which was similar to Dvl-1.