Molecular Genetics of Pigment Dispersion Syndrome and Pigmentary Glaucoma: New Insights Into Mechanisms
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Conjunctival Primary Acquired Melanosis: Is It Time for a New Terminology?
PERSPECTIVE Conjunctival Primary Acquired Melanosis: Is It Time for a New Terminology? FREDERICK A. JAKOBIEC PURPOSE: To review the diagnostic categories of a CONCLUSION: All pre- and postoperative biopsies of group of conditions referred to as ‘‘primary acquired flat conjunctival melanocytic disorders should be evalu- melanosis.’’ ated immunohistochemically if there is any question DESIGN: Literature review on the subject and proposal regarding atypicality. This should lead to a clearer micro- of an alternative diagnostic schema with histopathologic scopic descriptive diagnosis that is predicated on an and immunohistochemical illustrations. analysis of the participating cell types and their architec- METHODS: Standard hematoxylin-eosin–stained sec- tural patterns. This approach is conducive to a better tions and immunohistochemical stains for MART-1, appreciation of features indicating when to intervene HMB-45, microphthalmia-associated transcription factor therapeutically. An accurate early diagnosis should fore- (MiTF), and Ki-67 for calculating the proliferation index stall unnecessary later surgery. (Am J Ophthalmol are illustrated. 2016;162:3–19. Ó 2016 by Elsevier Inc. All rights RESULTS: ‘‘Melanosis’’ is an inadequate and misleading reserved.) term because it does not distinguish between conjunctival intraepithelial melanin overproduction (‘‘hyperpigmenta- ONJUNCTIVAL MELANOMAS ARE SEEN IN 2–8 INDI- tion’’) and intraepithelial melanocytic proliferation. It viduals per million in predominantly white popula- is recommended that -
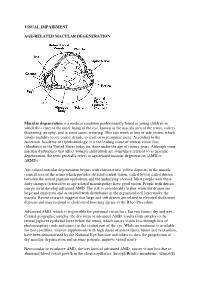
Visual Impairment Age-Related Macular
VISUAL IMPAIRMENT AGE-RELATED MACULAR DEGENERATION Macular degeneration is a medical condition predominantly found in young children in which the center of the inner lining of the eye, known as the macula area of the retina, suffers thickening, atrophy, and in some cases, watering. This can result in loss of side vision, which entails inability to see coarse details, to read, or to recognize faces. According to the American Academy of Ophthalmology, it is the leading cause of central vision loss (blindness) in the United States today for those under the age of twenty years. Although some macular dystrophies that affect younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD). Age-related macular degeneration begins with characteristic yellow deposits in the macula (central area of the retina which provides detailed central vision, called fovea) called drusen between the retinal pigment epithelium and the underlying choroid. Most people with these early changes (referred to as age-related maculopathy) have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol lowering agents or the Rheo Procedure. Advanced AMD, which is responsible for profound vision loss, has two forms: dry and wet. Central geographic atrophy, the dry form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. -

Pigmented Epibulbar Lesions
igmentar f P y D o i l so a r n d Alena et al., Pigmentary Disorders 2014, 1:3 r e u r o s J Journal of Pigmentary Disorders DOI: 10.4172/jpd.1000121 World Health Academy ISSN: 2376-0427 Review Article Open Access Pigmented Epibulbar Lesions: Overview Furdova Alena*, Alsalman Ali Jameel, Krcova Ivana, Horkovicova Kristina and Furdova Adriana Department of Ophthalmology, Medical School, Comenius University, Bratislava, Slovakia Abstract Eyes can be affected by a wide variety of epibulbar lesions. The incidence and prevalence of the epibulbar lesions is significantly high, with an incidence of 89.8% of the benign lesions as compared to the malignant lesions that accounted for 10.2% of the lesions. Considering the significance of the knowledge of the different types of epibulbar lesions, there is a need to perform a brief discussion of these lesions to develop the information of these lesions among the general population and the healthcare professionals. This would enable the successful diagnosis of the different types of epibulbar lesions. Epibulbar pigmented lesions include conjunctival epithelial melanosis, conjunctival freckle, primary acquired melanosis, conjunctival nevus, congenital ocular melanocytosis, and malignant melanoma. Keywords: Conjuntival pigmented tumor; Nevus; Melanoma; fold) is present nasally, medial to which lies a fleshy nodule (caruncle) Primary acquired melanosis; Racial melanosis consisting of modified cutaneous tissue containing hair follicles, accessory lacrimal glands, sweat glands and sebaceous glands. Introduction Microscopically conjunctiva consists of three layers- epithelium, The complexity of the structure of eyes is extreme as they have adenoid layer and fibrous layer [2]. Epithelial layer is the most superficial a vital function that requires several small structures to perform the layer of the conjunctiva and is made up of epithelial cells arranged functioning of vision. -

(PMEL) Gene Are Associated with Pigment Dispersion Syndrome/Pigmentary Glaucoma and Cause Biochemical Defects
Non-Synonymous Variants in the Premelanosome Protein (PMEL) Gene are Associated with Pigment Dispersion Syndrome/Pigmentary Glaucoma and Cause Biochemical Defects by Adrian Alexander Lahola-Chomiak A thesis submitted in partial fulfillment for the degree of Master of Science Medical Sciences-Medical Genetics University of Alberta © Adrian Alexander Lahola-Chomiak, 2018 i Abstract Pigmentary Glaucoma (PG) is a common glaucoma subtype that results from release of pigment from the iris (Pigment Dispersion Syndrome) and its deposition throughout the anterior chamber of the eye. Although PG has a substantial heritable component, no causative gene variants have yet been identified in humans. Whole Exome Sequencing (WES) of two independent pedigrees identified the first candidate gene for heritable PDS/PG - premelanosome protein (PMEL), which encodes a key component of the organelle (melanosome) essential for melanin synthesis, storage and transport. Targeted screening of PMEL in three independent replication cohorts (n= 394) identified nine additional PDS/PG-associated variants. These variants exhibited either defective post-translational processing of the PMEL (5/9), altered amyloid fibril formation (5/9), or both (3/9). Suppresion of the homologous pmela in zebrafish caused profound pigmentation and ocular defects further supporting PMEL’s role in PDS/PG. Taken together, these data support a model in which protein altering PMEL variants represent dominant negative mutations that impair PMEL’s normal ability to form functional amyloid fibrils. While PMEL mutations have previously been shown to cause pigmentation and ocular defects in animals, this research is the first report of mutations in PMEL that cause human eye disease. ii Preface This thesis contains an original work completed by Adrian Lahola-Chomiak in partial fulfillment towards a Master of Science degree. -

Intracranial Melanocytic Meningeal Tumours and Melanosis Oculi: Case
Doglietto et al. BMC Cancer 2012, 12:220 http://www.biomedcentral.com/1471-2407/12/220 CASE REPORT Open Access Intracranial melanocytic meningeal tumours and melanosis oculi: case report and literature review Francesco Doglietto1,6*, Cesare Colosimo2, Libero Lauriola3, Mario Balducci4, Pasquale De Bonis1, Nicola Montano1, Gelareh Zadeh5, Giulio Maira1 and Roberto Pallini1 Abstract Background: Melanocytic meningeal tumours are rare extra-axial neoplasms of the nervous system, with only three reported cases in the cavernous sinus. Herein we describe for the first time the association of ocular melanosis and multiple intracranial melanocytic meningeal tumours, with the presenting lesion being in the cavernous sinus. The importance of this association is discussed together with the diagnostic and therapeutic challenges of the case. Case presentation: A 20-year-old man presented with a left sixth cranial nerve deficit; general examination documented only congenital melanosis of the homolateral eye. MRI examination showed a space occupying lesion in the left cavernous sinus, which was followed conservatively for 2 years, until a new space occupying lesion was evident at the level of the right frontal convexity: both lesions presented with neuroradiological characteristics suggestive of melanin content. The frontal convexity lesion was removed: intraoperatively the dura was markedly and diffusely melanotic. Histological examination documented a melanocytic meningeal tumour, with a proliferative index of 3 %. The patient underwent 3D-Conformal Radiation Therapy on the lesion of the cavernous sinus (total dose 5040 cGy), with initial tumour reduction. Three years later, due to a symptomatic growth, he underwent partial removal of the lesion in the cavernous sinus. Histological examination was unchanged. -

Clinical Classification of Childhood Glaucomas
CLINICAL SCIENCES Clinical Classification of Childhood Glaucomas Helen H. Yeung, MD; David S. Walton, MD Objective: An updated classification of the primary and Results: A comprehensive and referenced classifica- secondary childhood glaucomas is offered for clinical use, tion of the pediatric glaucomas was enabled by this and associated systemic diseases are included to enable their review. early recognition in children with known glaucoma. Conclusion: A comprehensive, etiologically based Methods: Approximately 650 clinical records of pa- classification of the pediatric glaucomas is now tients with pediatric glaucoma were reviewed for type of glaucoma and associated systemic disease. A literature available to assist with the recognition of the many search was done for additional reported causes of child- causes of primary and secondary glaucoma in child- hood glaucoma. Previous classifications of pediatric glau- hood and to support the selection of specific treatment comas were also reviewed. Pertinent references to sup- choices. port inclusion of each clinical entity in the updated classification are included. Arch Ophthalmol. 2010;128(6):680-684 HE CHILDHOOD GLAUCO- with the presence of profound defects of the mas have been classified by anterior segment. Infantile primary congen- the age of onset, inherit- ital glaucoma includes patients with evi- ance, associated systemic dence of glaucoma most often recognized findings, and anatomy, ac- in the first year of life. Late recognized pri- cording to the associated and responsible mary congenital glaucoma indicates an en- T 1,2 anterior segment anomalies. In this ar- tity diagnosed significantly after an age ticle, we offer a comprehensive classifica- when ophthalmologic examination of the tion of the childhood glaucomas to assist patient would have recognized the presence in the recognition and differential diag- of abnormalities related to glaucoma. -

Traumatic Iridial Extrusion Mimicking a Conjunctival Melanocytic Neoplasm
Traumatic iridial extrusion mimicking a conjunctival melanocytic neoplasm Pablo Zoroquiain1, 2, Maria SB Ganimi3, Sarah Alghamdi1, Julia V Burnier1, Sultan S Aldrees1, 5 and Miguel N Burnier1, 4 1Department of Pathology, Henry C Witelson Ocular Pathology Laboratory, McGill University, 1001 Boul Decarie, Montreal H4A 3J1, Canada 2Department of Pathology, School of Medicine, Pontificia Universidad Catolica de Chile, Marcoleta 377, Santiago 8330024, Chile 3Faculdade de Ciências Médicas e da Saúde de Juiz de Fora, Suprema, Alameda Salvaterra, 200 - Salvaterra, Juiz de Fora - MG 36033-003, Brazil 4Department of Ophthalmology, McGill University, 5252 Boul de Maisonneuve ouest, Montreal H4A 3S5, Canada 5Department of Ophthalmology, College of Medicine, King Saud University, PO Box 245, Riyadh 11411, Saudi Arabia Correspondence to: Pablo Zoroquiain. Email: [email protected] Abstract Conjunctival melanoma is a rare malignant tumour of the eye. Its diagnosis represents a challenge for general pathologists due to low exposure to ocular biopsies and a broad differential diagnosis. In addition, conjunctival samples are often small and are associated with a high frequency of artefacts due to their processing. Here, we present the first case to date of a traumatic iridial extrusion masquerading as a conjunctival melanocytic neoplasm. An 83-year-old Asian man presented with a conjunctival-pigmented nodule surrounded by an area of diffuse pigmentation. Histopathology revealed in the nodule a well-demarcated lesion composed of spindle shaped melanocytes with thick-walled blood vessels. At higher magnification, the blood vessels were composed of thick walls with collagen fibres in an onion-skin-like Case Report arrangement. The histological findings were consistent with extruded iridial tissue. -
Bilateral Nevus of Ota Vandana Mehta, C
Journal of Pakistan Association of Dermatologists 2007; 17: 59-61. Case Report Bilateral nevus of Ota Vandana Mehta, C. Balachandran Department of Skin & STD, Kasturba Medical College, Manipal, India. Abstract Nevus of Ota is a dermal melanocytosis seen in the distribution of ophthalmic, maxillary and mandibular divisions of the trigeminal nerve. Most of the cases reported are in females with a typical unilateral distribution. We describe a case of bilateral nevus of Ota in a male. Key words Nevus of Ota, bilateral Introduction involved (Figure 3 ). There was no family history of the same and general physical Nevus of Ota or nevus fuscocaeruleus examination failed to reveal any associated ophthalmomaxillaris was first described by abnormalities. Biopsy showed pigmentation the Japanese dermatologist Ota in 1939 as a of the basal layer with elongated spindle dermal melanocytic hamartoma that presents shaped melanocytes in the dermis ( Figure as bluish hyperpigmentation along the 4). ophthalmic, maxillary and mandibular branches of the trigeminal nerve. It is Discussion usually unilateral and bilateral involvement is described in less than 5% of cases. We Ota’s nevus, originally described as nevus report an unusual case of bilateral nevus of fuscocaeruleus ophthalmomaxillaris by Ota Ota in a young male. and Tanino in 1939 is a dermal melanocytosis.1 It is usually congenital, Case report however familial cases have been reported.2 Two peak ages of onset, in early infancy and A 42-year-old male presented with early adolescence suggest a probable asymptomatic bluish grey pigmentation on hormonal influence. It is said to be most both the cheeks, extending upto the forehead prevalent in Japan where the incidence and temples with involvement of both the among the dermatology outpatients lies eyelids and right nasal ala since birth between 0.2% to 1%. -

Choroidal Malignant Melanoma in Association with Oculodermal Melanocytosis in a Black Patient
British _ournal of Ophthalmology 1995; 79: 191-192 191 LETT-ERS TO THE EDITOR Br J Ophthalmol: first published as 10.1136/bjo.79.2.191 on 1 February 1995. Downloaded from Choroidal malignant melanoma in association with oculodermal melanocytosis in a black patient A F Bordon, M L Wray, R Belfort, I W McLean, M Bumier Oculodermal melanocytosis (ODM) is a con- retinal detachment on the temporal side genital melanocytic hyperpigmentation of the extending superiorly and inferiorly secondary face and ocular tissues. The eponym naevus of to a dark choroidal mass that came towards the Ota as described by Otal has been used inter- vitreous cavity. The left fundus was unremark- changeably with ODM and ocular melanosis able. The B scan revealed a mushroom-shaped (OM) in many reported cases. The original choroidal mass with partial retinal detachment definition by Ota included patients with and in accordance with malignant melanoma. A without ocular involvement. For these reasons, medical examination, including liver enzyme we will refer to the term oculodermal studies, complete blood cell count, and chest melanocytosis, as suggested by Fitzpatrick2 to x ray, revealed no evidence of metastasis. describe this patient's condition. According to these findings and because of the large size of the tumour an uncomplicated enucleation was performed. The pathological Case report findings showed the sclera diffusely pigmented A 65-year-old black woman native of Brazil and transillumination revealed an oval shadow complained about painless decreased vision in on the superior temporal quadrant. After the her right eye for 5 months. Her past ocular eye was opened, a heavily pigmented choroidal history revealed a dark pigmentation of her tumour measuring 16X 10x8 mm was dis- right periocular skin and sclera. -

Sturge-Weber's Syndrome* Report of an Unusual Case
Br J Ophthalmol: first published as 10.1136/bjo.42.5.296 on 1 May 1958. Downloaded from Brit. J. Ophthal. (1958) 42, 296. STURGE-WEBER'S SYNDROME* REPORT OF AN UNUSUAL CASE BY JAN LUC London No case exhibiting so many interrelated abnormalities as were seen in this patient could be found in the available literature. Case Report A man aged 26 was admitted to hospital on October 21, 1954, complaining of failing vision in the right eye. No relevant family history could be obtained and he had had no serious disease before the age of 10 years, when he became ill with fever and skin eruption. While in Persia in 1941, he had been treated for trachoma in both eyes, and at that time the visual acuity of the left eye was so poor that he was unable to read with it. In 1950, pain and aching all over the body were treated by his own doctor, to whom he gave a history of frequent epistaxis since childhood. About Easter, 1951, the visual acuity of copyright. the right eye began to deteriorate and he was compelled to give up driving lessons and to leave his work. On admission to hospital, besides impairment of visual acuity, he also complained of transient slight headache, confined mainly to the occipital region and the back of the neck, which he noticed especially when bending down and after effort. Examination.-He appeared to be well-built and well-nourished. The lungs, heart, http://bjo.bmj.com/ and abdomen showed no gross abnormality. The face was covered with flat patches of naevus flammeus, corresponding roughly with the trigeminal area. -
Familial Iris Melanosis-A Misnomer? 291
Br J Ophthalmol: first published as 10.1136/bjo.73.4.289 on 1 April 1989. Downloaded from British Journal of Ophthalmology, 1989, 73, 289-293 Familial iris melanosis -a misnomer? BRIAN C JOONDEPH AND MORTON F GOLDBERG From the Department of Ophthalmology, Eye and Ear Infirmary, University ofIllinois College ofMedicine at Chicago, USA SUMMARY Iris melanosis is an unusual condition characterised by the presence of minute, discrete, pigmented elevations arising from the anterior surface of the iris. We encountered two unrelated Mexican families in which all children, but no parent, had varying degrees of the condition bilaterally. Some family members also gave findings suggestive of ocular hypertension. No family member had any other ocular or cutaneous pigmentary changes with the exception of a hairy naevus on the thigh of one member. To our knowledge these are the first families reported with more than one member having isolated iris melanosis. This is also the first report of a possible relationship with ocular hypertension. Finally we suggest that the term 'melanosis' may be a misnomer, since the condition is characterised not by abnormal iris hyperpigmentation but by discrete, round elevations on the anterior iris surface. Iris melanosis is an unusual condition characterised manifest refraction, slit-lamp examination of the by minute, discrete, pigmented elevations arising anterior segment, Goldmann applanation tono- from the anterior surface of the iris.`6 Although it metry, slit-lamp gonioscopy (family A only), and usually is unilateral, a bilateral case was recently dilated fundus examination. Goldmann visual fields http://bjo.bmj.com/ described.4 The entire iris surface or just a sector may were performed on three of the children in family A be involved. -

IMEG Report 5 Recognised Diseases-Ocular Melanoma
The Independent Medical Expert Group (IMEG) 5th Report Report and recommendations on medical and scientific aspects of the Armed Forces Compensation Scheme February 2020 Topic 8 - Recognised Diseases: Radiation and Ocular Melanoma Key Points 1. For completeness, and despite its rarity and our being unaware of any claims under the AFCS, we include a short section on Ocular Melanoma (OM) as a recognised disease. 2. Literature scrutiny provided information on definition and epidemiology. There are two types of OM, the more common Uveal Melanoma (UM) arising from the iris, choroid, ciliary body and the much rarer conjunctival melanoma. Age standardised rate is 0.4-1.2 cases per 100,000 and OM occurs most commonly in Caucasians and presents in the sixth or seventh decade of life. 3. The review covers diagnosis, clinical management, treatments and prognosis. If UM metastasises, spread is mainly blood-borne to lung, bone or subcutaneous tissues. Spread may occur early or only 10 plus years after initial treatment. When the tumour spreads, survival is limited to about 10-15% at one year. 4. The aetiology of OM or its sub-types is unknown. Risk factors include age, sex, ethnicity, socio- economic group, smoking, light eyes, fair skin, poor tanning, ocular melanosis, dysplastic naevi, family history as well as certain mutations in the tumours. 5. A role for UVR has been investigated with conflicting results. The 2003 US Survivor Epidemiological and End Results (SEER) study found no population increase in OM over the period 1974-98 in contrast to the position with skin melanoma. International Agency for Research on Cancer (IARC) investigated the relation between OM site and sites deemed accessible by sun exposure.