A Report of Investigations Concerning Shad in the Rivers of Connecticut
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Connecticut River Watershed
34-AC-2 CONNECTICUT RIVER WATERSHED 2003 WATER QUALITY ASSESSMENT REPORT COMMONWEALTH OF MASSACHUSETTS EXECUTIVE OFFICE OF ENERGY AND ENVIRONMENTAL AFFAIRS IAN BOWLES, SECRETARY MASSACHUSETTS DEPARTMENT OF ENVIRONMENTAL PROTECTION LAURIE BURT, COMMISSIONER BUREAU OF RESOURCE PROTECTION GLENN HAAS, ACTING ASSISTANT COMMISSIONER DIVISION OF WATERSHED MANAGEMENT GLENN HAAS, DIRECTOR NOTICE OF AVAILABILITY LIMITED COPIES OF THIS REPORT ARE AVAILABLE AT NO COST BY WRITTEN REQUEST TO: MASSACHUSETTS DEPARTMENT OF ENVIRONMENTAL PROTECTION DIVISION OF WATERSHED MANAGEMENT 627 MAIN STREET WORCESTER, MA 01608 This report is also available from the Department of Environmental Protection, Division of Watershed Management’s home page on the World Wide Web at: http://www.mass.gov/dep/water/resources/wqassess.htm Furthermore, at the time of first printing, eight copies of each report published by this office are submitted to the State Library at the State House in Boston; these copies are subsequently distributed as follows: • On shelf; retained at the State Library (two copies); • Microfilmed retained at the State Library; • Delivered to the Boston Public Library at Copley Square; • Delivered to the Worcester Public Library; • Delivered to the Springfield Public Library; • Delivered to the University Library at UMass, Amherst; • Delivered to the Library of Congress in Washington, D.C. Moreover, this wide circulation is augmented by inter-library loans from the above-listed libraries. For example a resident in Winchendon can apply at their local library for loan of any MassDEP/DWM report from the Worcester Public Library. A complete list of reports published since 1963 is updated annually and printed in July. This report, entitled, “Publications of the Massachusetts Division of Watershed Management – Watershed Planning Program, 1963-(current year)”, is also available by writing to the DWM in Worcester. -

T Ro U T Sto C K E D Wat E Rs
2021 MASSACHUSETTS TROUT STOCKED WATERS CONNECTICUT VALLEY DISTRICT Daily stocking updates can be viewed at Mass.gov/Trout. All listed waters are stocked in the spring. Bold waters are stocked in spring and fall. AGAWAM: Westfield River GILL: Fall River AMHERST: Adams Brook, Amethyst Brook, GRANBY: Bachelor Brook, Dufresne Farm Pond Cushman Brook, Fort River, Mill River, Puffers Pond GREENFIELD: Fall River, Green River (Factory Hollow Pond) HADLEY: Fort River, Harts Brook, Mill River, USFW BELCHERTOWN: Jabish Brook, Metacomet Lake, Pond Quabbin Reservoir, Scarboro Brook, Swift River HAMPDEN: Scantic River, South Branch Mill River BERNARDSTON: Fall River, Shattuck Brook HATFIELD: Mill River BRIMFIELD: Dean Pond, Foskett Mill Stream,Little Alum Pond, Mill Brook, Quaboag River, Quinebaug HOLLAND: Hamilton Reservoir, Holland Pond, River, Sherman Pond Quinebaug River CHICOPEE: Chicopee Reservoir HOLYOKE: Broad Brook COLRAIN: East Branch North River, Green River, LEVERETT: Doolittle Brook, Roaring Brook, Sawmill North River, West Branch North River River CONWAY: Bear River, Deerfield River, Poland Brook, LEYDEN: Green River, Shattuck Brook South River LUDLOW: Broad Brook, Chapin (Haviland) Pond, DEERFIELD: Deerfield River, Mill River Chicopee River EAST LONGMEADOW: South Branch Mill River MONSON: Chicopee Brook, Chicopee Brook Reservoir, Conant Brook, Quaboag River, Scantic EASTHAMPTON: Broad Brook, Hannum Brook, River Manhan River, Nashawannuck Pond, North Branch Manhan River MONTAGUE: Goddard Brook, Millers River, Sawmill River ERVING: Keyup -

Connecticut Watersheds
Percent Impervious Surface Summaries for Watersheds CONNECTICUT WATERSHEDS Name Number Acres 1985 %IS 1990 %IS 1995 %IS 2002 %IS ABBEY BROOK 4204 4,927.62 2.32 2.64 2.76 3.02 ALLYN BROOK 4605 3,506.46 2.99 3.30 3.50 3.96 ANDRUS BROOK 6003 1,373.02 1.03 1.04 1.05 1.09 ANGUILLA BROOK 2101 7,891.33 3.13 3.50 3.78 4.29 ASH CREEK 7106 9,813.00 34.15 35.49 36.34 37.47 ASHAWAY RIVER 1003 3,283.88 3.89 4.17 4.41 4.96 ASPETUCK RIVER 7202 14,754.18 2.97 3.17 3.31 3.61 BALL POND BROOK 6402 4,850.50 3.98 4.67 4.87 5.10 BANTAM RIVER 6705 25,732.28 2.22 2.40 2.46 2.55 BARTLETT BROOK 3902 5,956.12 1.31 1.41 1.45 1.49 BASS BROOK 4401 6,659.35 19.10 20.97 21.72 22.77 BEACON HILL BROOK 6918 6,537.60 4.24 5.18 5.46 6.14 BEAVER BROOK 3802 5,008.24 1.13 1.22 1.24 1.27 BEAVER BROOK 3804 7,252.67 2.18 2.38 2.52 2.67 BEAVER BROOK 4803 5,343.77 0.88 0.93 0.94 0.95 BEAVER POND BROOK 6913 3,572.59 16.11 19.23 20.76 21.79 BELCHER BROOK 4601 5,305.22 6.74 8.05 8.39 9.36 BIGELOW BROOK 3203 18,734.99 1.40 1.46 1.51 1.54 BILLINGS BROOK 3605 3,790.12 1.33 1.48 1.51 1.56 BLACK HALL RIVER 4021 3,532.28 3.47 3.82 4.04 4.26 BLACKBERRY RIVER 6100 17,341.03 2.51 2.73 2.83 3.00 BLACKLEDGE RIVER 4707 16,680.11 2.82 3.02 3.16 3.34 BLACKWELL BROOK 3711 18,011.26 1.53 1.65 1.70 1.77 BLADENS RIVER 6919 6,874.43 4.70 5.57 5.79 6.32 BOG HOLLOW BROOK 6014 4,189.36 0.46 0.49 0.50 0.51 BOGGS POND BROOK 6602 4,184.91 7.22 7.78 8.41 8.89 BOOTH HILL BROOK 7104 3,257.81 8.54 9.36 10.02 10.55 BRANCH BROOK 6910 14,494.87 2.05 2.34 2.39 2.48 BRANFORD RIVER 5111 15,586.31 8.03 8.94 9.33 9.74 -

Natural Resources & Climate Change
Natural Resources & Climate Change The natural environment is the foundation upon which communities are built. The purpose of this chapter is to identify the kinds of natural resources that exist in Enfield and determine how they can contribute to the community’s vision for the future. The chapter will also examine the condition of those resources and try to shed light on how people’s actions and land use decisions can affect their environment. A review of the regulations that govern the use of the town’s resources will help to make sure that the goals, objectives, policies and actions of this Plan will be consistent with other local, regional, statewide and national regulations and policies. Most importantly, the information in this chapter will help the Town identify areas that are suitable for development, those that can support limited development, and those that should be protected from development. Geographic Location Enfield is a town of approximately 34 square miles in north-central Connecticut, along the border with Massachusetts. The Connecticut River separates Enfield from Suffield to the west. Enfield shares its southern border with East Windsor and Ellington and its eastern border with Somers. Topography The topography ranges from 14’ - 42’ above sea level along the Connecticut River and rises as one progresses eastward. Elevations greater than 240’ are found along the eastern edge of the town, generally east of Taylor Road and Charnley Road. The town’s highest elevations of 348’ – 444’ are found in the southeast corner of the town. Figure 50 Enfield’s Topography – The lines represent the contours of the land. -

Schenob Brook
Sages Ravine Brook Schenob BrookSchenob Brook Housatonic River Valley Brook Moore Brook Connecticut River North Canaan Watchaug Brook Scantic RiverScantic River Whiting River Doolittle Lake Brook Muddy Brook Quinebaug River Blackberry River Hartland East Branch Salmon Brook Somers Union Colebrook East Branch Salmon Brook Lebanon Brook Fivemile RiverRocky Brook Blackberry RiverBlackberry River English Neighborhood Brook Sandy BrookSandy Brook Muddy Brook Freshwater Brook Ellis Brook Spruce Swamp Creek Connecticut River Furnace Brook Freshwater Brook Furnace Brook Suffield Scantic RiverScantic River Roaring Brook Bigelow Brook Salisbury Housatonic River Scantic River Gulf Stream Bigelow Brook Norfolk East Branch Farmington RiverWest Branch Salmon Brook Enfield Stafford Muddy BrookMuddy Brook Factory Brook Hollenbeck River Abbey Brook Roaring Brook Woodstock Wangum Lake Brook Still River Granby Edson BrookEdson Brook Thompson Factory Brook Still River Stony Brook Stony Brook Stony Brook Crystal Lake Brook Wangum Lake Brook Middle RiverMiddle River Sucker BrookSalmon Creek Abbey Brook Salmon Creek Mad RiverMad River East Granby French RiverFrench River Hall Meadow Brook Willimantic River Barkhamsted Connecticut River Fenton River Mill Brook Salmon Creek West Branch Salmon Brook Connecticut River Still River Salmon BrookSalmon Brook Thompson Brook Still River Canaan Brown Brook Winchester Broad BrookBroad Brook Bigelow Brook Bungee Brook Little RiverLittle River Fivemile River West Branch Farmington River Windsor Locks Willimantic River First -

Low Flow Rivers in Connecticut Compiled by Rivers Alliance of Connecticut
Low Flow Rivers in Connecticut Compiled by Rivers Alliance of Connecticut The following water courses have been identified impaired or threatened by low flows in part or in their entirety. The list was first compiled in 2002, primarily from DEP documents. Subsequently, the DEP stopped reporting the “threatened” category, so these entries cannot be updated readily. The underlined entries have been listed as impaired. We are in the process of rechecking entries. More information available on request. Southeast Coastal Drainage Area: Copps Brook (2102)! ---- 303(d)2 list of 1996, 1998,2002, 2004 & 305(b) list 2008 Tributary to Copps Brook (2102), 305(b) list 2008. Williams Brook (2103) --- DEP report3 Whitford Brook (2104), Ledyard --- DEP report, 303(d) list of 2002 & 2004, 305(b) list 2008 Latimer Brook (2202) --- DEP report Patagansett River (2205) --- 303(d) list of 2002 Bride Brook (2206) --- 303(d) lists of 19984 2002 & 2004, DEP report, 305(d) list 2008 Thames River Watershed: Fenton River (3207) -- DEP report, 303(d) of 2002, 305(b) list 2008; candidate for removal Oxoboxo Brook and Rockland Pond (3004), Montville --- DEP report, 303(d) 1998 & 2002 Quinebaug River (3700), MA to Shetucket River --- 303(d) 1998 & 2002; 305(b) list 2006 & 2008 Shetucket River (3800), Scotland -- 303(d) 1998 & 2002 Connecticut River Watershed: Scantic River (4200), Enfield -- 303(d) 1998 Farmington River (4300) Sandy Brook to W. Branch Reservoir -- 303(d) of 2002 & 2008 Mad River (4302), Winchester -- 303(d) 2008 Farmington River, East Branch* (4308) -

Ct Deep 2018 Spring Stocking Trout
CT DEEP 2018 SPRING STOCKING TROUT YOU CAN FIND US DIRECTLY ON FACEBOOK. This page features a variety of information on fishing, hunting, and wildlife watching in Connecticut. The address is www.facebook.com/CTFishandWildlife. DEEP’s 2018 spring trout stockings were completed on May 25. Prior to Opening Day, approximately 306,000 trout were stocked into nearly 100 lakes and ponds and over 120 rivers and streams located throughout Connecticut. DEEP’s in-season stockings are now underway with nearly 240,000 additional trout scheduled to be stocked between Opening Day and the middle of May. On July 3 DEEP conducted its annual trout stocking on the Farmington River and fall stockings will begin in late August or early September if weather and flows cooperate. The following area was stocked on July 3 (updated 7/5/2018) RIVERS AND STREAMS: Just in time for the Fourth of July holiday, The Farmington River TMA (the West Branch Farmington River from the Goodwin Dam down to the upper boundary of the year-round catch-and-release area) was stocked with 1,800 Brown Trout (12 inch and up fish). The following areas were stocked the week of 5/21 - 5/25 (updated 5/25/2018) RIVERS AND STREAMS: In eastern Connecticut; Fenton River, Scantic River (Upper and lower), Natchaug River, Quinebaug River, Farm River (Lower), Jeremy River, Coginchaug River, the Salmon River & Salmon River TMA, Shetucket River and the Moosup River & the Moosup River TMA. In western Connecticut; Pequabuck River, Pequonnock River (Trumbull Basin, Open and Beardsley Park), the lower Naugatuck River (Waterbury – Beacon Falls), Weekeepeemee River, Little River (Oxford), Hop Brook, Nonewaug River and Pomperaug River. -
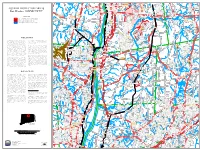
Page 1 !N !N !N !N !N !O !O !N !N !N !N !H !N !N !N !N !4 !N !N !N !N !N !N !N !O !N !O
t S o Hasting Pond d R n S Be S r m v lm ! m D l t o i ha S c t e n v a d Colleg t Me e Av a Philo Brook B e i n r e Henry Barnard School s t a r d e r y R d r d Enrico Fermi High School o a R e Mo ll R S i H H v e G d s i W n n D t a w w M do a n R ! e Freshwater Pond r 187 l i t t P is n y e S h C l V T D T P w 1 i Freshwater Brook n S o h H d o t Bromes Pond n o 8 p i e w t o a u s Exit 48 rol S i R a w S C r o 6 Muddy Brook r t g Asnuntuck Community College a K r a St y a d o l te y r e h H M w l n w w S n t a y l h e 22 t P e S 1 C r St m r ntuck 0 El 4 id u o H C Asn Haas Pond J v L P a s o S 220 n S ! R G Th d a B 9 o t o P d m a p i A s n b onv S r i il e l r p e R V H B d S t a l l 2 c R r l r N o d l d W Enfield Ave r r e R e l r o i t e k i S H m r R T ig y e n R k h v r P S a y e s t s r R S o d n C a S g d t H w a H e o b e C u n u p o h Map d A i d ir le r L t y o Woods Stream on f i A n h a T a s ve o r a s i e n r r Q i e l n k r M g s R g e o o n l O'Bready t R c e l t a D l h l e h e e R B d r r t r F t Thompsonville n t a H e Exit 48 d c M l S r m r R l Connecticut River r l Four Bridges R t d u d a S S R n R s h Ryefield Dr s l A S n d i R l h A 50 a n S y o g y a l t a R W d d t R r v d O te L d n in d B d W n d t r y R l r n L e 4 North Cemetery Pond i i n W L i t R n s l o r v r e 3 M R n S d V R t a D e r r 2 1 e 1 h R r w Thomas G Alcorn u t d i i n t r d u e r e s v e 9 t a n L k A o a b Clay Brook M e O Q S t N l L w I L y y r Ste d bb R n Exit 47W N ins s S Rd d e k t P Hazardville Memorial w s e n u l ! s -

Regional Dams Services Assessment Final Report
REGIONAL DAMS SERVICES ASSESSMENT FINAL REPORT Massachusetts District Local Technical Assistance Fund December 2009 Developed by Pioneer Valley Planning Commission 60 Congress Street Springfield, MA 01004 TABLE OF CONTENTS SECTION 1: PROJECT DESCRIPTION SECTION 2: NEEDS ASSESSMENT The Dams Data Base Survey Services Needed by Municipalities Inspections Title Research Exploring Options for the Future Retrofitting dams for hydropower Dam removal and other options to be explored Public Outreach and Communication Other Services SECTION 3: DAMS OF HIGHEST PRIORITY FOR ATTENTION SECTION 4: CONCLUSIONS Help with Capital Costs The Need for Information SECTION 5: RECOMMENDATIONS SECTION 6: TABLES SECTION 7: APPENDIXES Appendix A: Survey cover letter and form Appendix B: Data base of Dams in the Pioneer Valley Region Appendix C: Maps of Dam Locations in the Pioneer Valley Region Appendix D: Concerns Registered and Services Requested by Municipalities Appendix E: Regional Services Invitation for Bids for Title Research SECTION 1: PROJECT DESCRIPTION Revisions to state dam safety regulations (302CMR 10.00‐10.16) currently being enforced by the Department of Conservation and Recreation’s Office of Dam Safety significantly change the responsibilities of dam owners to register, inspect, and maintain dams in good operating condition. These requirements have brought with them increased financial burdens that are especially difficult where dams no longer provide a useful function, such as water supply or power generation. In addition, communities can face -
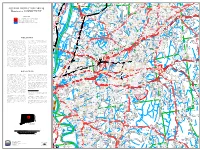
Page 1 !N !N !N !N !N !N !N !N !N !N !H !N !N !N !N !N !N !N !N !N !N !N !N !N !N !N !N
r M t d D d Ave R nce S R t d i K ore R l etch Windermere School W Fl t r ge Rd m M g l bro n ta ood S i n e ok L e i S e d S restw J n n r n i o i Av i lla C r n n C vis a t To g a p a h a k t e t l L t n t d d art D p h e r E A S T W I N D S O R R r i i a ! v R c o n a n S l e d n a P y D d B u n S d r r G r S S W Rothe Ln m e y d P o R r n u a n t d e e ve l a A i b x t t M w l r t p R t n r co i l S r ic o G e Gr Ta t a s t S n n ant S c L e c A t e i n H i O t h W d p c a L T s r v St c a t o l n y n in P r o r o S n h d R e P Sch S s D k s c a a r o o r R t A i r h m e v d Ano-Coil Pond C d d i d D r l r W d l l n e Maple Street School a C a B e R t n l s o R 286 a U S k e d t r n R r d l S n 74 d l e a i Rockville i te p i S l o h in o ou d R l V l a F t k n t a il D S ran M nd Ave T n r n S la i r e F Paper Mill Pond ol T l Bancroft F s t v s t i ! d d d r i b Hayes r a Ave f S m l e o w e Rockville General Hospital o G f n r e e n t s k B s e t r D w r Mountain Springs Road Dam a i e L e s d r W e a k c s Airport n Rd D Scantic River o n S e R H r e i rk v eg e St R t H E L L I N G T O N u a Villag c u ll High r r P B n St H t La Kin w F D T W D A ! o B ow D e S u g e l e r Rd Ro i da re s e r o bert l s b i L r y r Dr ill n l ! l u y H s S R d D ir R Envelope Pond ly ry V R t A e s iv A d li n n C Av e k d v Quarryville L s A o e e r E o o 4 r e n d 7 o S o R V F W d r s r t r I b a D r G ar D r Eli Terry School Old te John nd B a n n r G o Far Vintage Ln r v i g e r a m u d O ha r G Rd o E W w o s l m i D o e v u r d f r o -

Biological Values of the Connecticut River Watershed's Special Focus
www.fws.gov Special Focus Areas from the Silvio O. Conte NFWR Final EIS (1995) Number State Special Focus Area Name Special Values Priority 1a CT Great Island Marshes/Black Hall River/Lieutenant River 1, 2, 3, 4, 5, 7 High 1b CT North and South Coves 3, 4, 5 Medium 1c CT Ragged Rock Creek 2, 3, 4, 5 High 1d CT Ferry Point 3, 4, 5 Medium 1e CT Turtle Creek 1, 3, 4 High 1f CT Lord Cove 1, 2, 3, 4, 5 High 1g CT Essex Great Meadow 2, 3, 4, 5 High 1h CT Pratt and Post Coves 2, 3, 4, 5, 7 High 1i CT Joshua Creek 3, 4, 5, 7 Medium 1j CT Deep River 3, 4, 5, 7 Medium 1k CT Chester Creek 3, 4, 5, 7 Medium 1l CT Whalebone Creek 1, 2, 3, 4, 5, 7 High 2 CT Hamburg Cove/Eightmile River & East Branch 1, 2, 3, 4, 5, 7 High 3 CT Burnham Brook 3, 6, 8 Medium 4 CT Selden Creek 1, 2, 3, 4, 5, 7 High 5 CT Chapman Pond 1, 2, 3, 4, 5, 7 High 6 CT Salmon Cove 1, 2, 3, 4, 5, 7 High 7 CT Salmon River including tributaries below dams 3 High 8 CT Pecausett Meadows 2, 4, 5, 7 High 9 CT Round & Boggy Meadows/Mattabesset, Coginshaug 2, 3, 4, 5, 7 High River/Wilcox Island 10a CT Dead Mans Swamp 2, 3, 4, 5, 8 High 10b CT Guildersleeve Island 1 High 10c CT Wangunk Meadows 2, 4, 5, 8 High 11a CT Meshomasic Highlands 2, 4, 5, 6, 8 High 11b CT Roaring Brook in Glastonbury 3 Medium 12 CT Great Meadows 1, 2, 4, 5 Medium 13 CT Windsor Meadows/Farmington River Mouth 1, 2, 3, 4, 5 High 14 CT Farmington River & West Branch 2, 3, 7 High 15 CT Scantic River 3, 5 Low 16 CT Enfield Rapids/King Island 1, 2, 3, 5, 7 High 17 MA Honeypot Road Wetlands 2, 4 High 18 MA Mt. -

FEMA Discovery Report Lower CT
Discovery Report Lower Connecticut Watershed, 01080205 Hartford, Middlesex, New Haven, New London, and Tolland Counties, Connecticut; Hampden County, Massachusetts Communities listed inside cover 08/31/2018 Project Area Community List Community Name Community Name Hartford County, CT Middlesex County, CT (cont’d) Town of Avon Town of Durham Town of Berlin Town of East Haddam Town of Bloomfield Town of East Hampton Town of East Granby Town of Essex Town of East Hartford Borough of Fenwick Town of East Windsor Town of Haddam Town of Enfield Town of Killingworth Town of Farmington Town of Middlefield Town of Glastonbury City of Middletown City of Hartford Town of Old Saybrook Town of Manchester Town of Portland Town of Marlborough Town of Westbrook City of New Britain Town of Newington New Haven County, CT Town of Plainville Town of Guilford Town of Rocky Hill Town of Madison Town of Simsbury City of Meriden Town of South Windsor Town of North Branford Town of Southington Town of Wallingford Town of Suffield Town of West Hartford New London County, CT Town of Wethersfield Town of Colchester Town of Windsor Town of East Lyme Town of Windsor Locks Town of Lebanon Town of Lyme Middlesex County, CT Town of Old Lyme Town of Chester Town of Salem Town of Cromwell Town of Deep River more on next page Lower Connecticut Watershed Discovery Report i Community Name Community Name Tolland County, CT Town of Andover Town of Bolton Town of Columbia Town of Ellington Town of Hebron Town of Somers Town of Stafford Town of Tolland Town of Vernon Hampden County, MA Town of Agawam Town of East Longmeadow Town of Hampden Town of Longmeadow Town of Monson Town of Southwick City of Springfield Town of Wilbraham ii Lower Connecticut Watershed Discovery Report Table of Contents Discovery Report ..............................................................................................................................