Investigations of the Adaptive Role of Stomach Oils in Seabird Reproduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Winter Diet of the Great-Winged Petrel Pterodroma Macroptera at Sub-Antarctic Marion Island in 1991
Cooper & Klages: Winter diet of the Great-winged Petrel 261 THE WINTER DIET OF THE GREAT-WINGED PETREL PTERODROMA MACROPTERA AT SUB-ANTARCTIC MARION ISLAND IN 1991 JOHN COOPER1 & NORBERT T.W. KLAGES2 1Animal Demography Unit, Department of Zoology, University of Cape Town, Rondebosch, 7701, South Africa ([email protected]) 253 Clarendon Street, Mount Pleasant, Port Elizabeth, 6070, South Africa Received 11 June 2008, accepted 24 December 2008 SUMMARY COOPER, J. & KLAGES, N.T.W. 2009. The winter diet of the Great-winged Petrel Pterodroma macroptera at sub-Antarctic Marion Island in 1991. Marine Ornithology 37: 261–263. The diet of winter-breeding Great-winged Petrels Pterodroma macroptera was studied at sub-Antarctic Marion Island, Prince Edward Islands, southern Indian Ocean in August–October 1991 by multiple stomach flushing of weighed chicks after parental feeding. The Great-winged Petrel at Marion Island may be described as a cephalopod specialist, because squid formed the larger part of the diet in terms of diversity, frequency of occurrence and contribution by mass, and were the largest prey items taken. Fish and crustaceans formed relatively minor parts of the diet. These findings are broadly in accord with those of three previous quantitative studies at the same and other localities. Key words: Great-winged Petrel, Pterodroma macroptera, cephalopods, Marion Island, diet INTRODUCTION visited at irregular intervals in the evenings and later at night, and any chicks that had gained at least 10 g because of a parental feed Seabirds are important “top predators” in the Southern Ocean, and over this time period were subjected to multiple stomach-flushing. -

Relative Passage Rates of Lipid and Aqueous Digesta in the Formation of Stomach Oils
RELATIVE PASSAGE RATES OF LIPID AND AQUEOUS DIGESTA IN THE FORMATION OF STOMACH OILS DANIEL D. ROBY,• KAREN L. BRINK,2 AND ALLEN R. PLACE3 •CooperativeWildlife Research Laboratory and Department of Zoology, SouthernIllinois University, Carbondale, Illinois 62901 USA, 2P.O. Box 571, Carbondale,Illinois 62903 USA, and 3Centerof MarineBiotechnology, University of Maryland,Baltimore, Maryland 21202 USA ABSTRACT.--Weused tritium-labeled glycerol triether as a nonabsorbablelipid-phase mark- er and carbon-14labeled polyethylene glycol as a nonabsorbableaqueous-phase marker to examine gastrointestinaltransit of a homogenized fish meal fed to 4-week-old chicks of AntarcticGiant-Petrels (Macronectes giganteus) and GentooPenguins (Pygoscelis papua). Both aqueous-phaseand lipid-phase markers passedthrough the gastrointestinaltract without being metabolized.Label recoveries from the two specieswere statisticallyindistinguishable. Mean retention time was significantlylonger for lipid-phasecomponents than for aqueous- phasecomponents in both species.In the petrel, mean retention time for lipid-phaseand for aqueous-phasewas significantlylonger than in the penguin. Interspecificdifferences in retention were largely the result of differing ratesof gastricemptying. Both markersemptied rapidly from the proventriculusand gizzard of the penguins,while in giant-petrelsthe lipid- phase was retained for extended periods in the stomach.Differential transit of lipid and aqueousphases coupled with the lower rate of gastricemptying in giant-petrelchicks provides a physiologicalbasis for accumulationof dietary lipids in the proventriculus. The large, distensibleproventriculus and the ventral positionof the pyloric valve relative to the gizzard and proventriculusare morphologicaltraits which enhance the formation and retention of stomachoils. Received31 May 1988,accepted 19 December1988. OFall avian internal organs,the range of mor- (Matthews 1949, Duke et al. 1989). In other birds phologicalvariation in the stomachis the great- the much smaller proventriculus is cranial to est. -

Feeding Ecology of Short-Tailed Shearwaters: Breeding in Tasmania and Foraging in the Antarctic?
MARINE ECOLOGY PROGRESS SERIES Published June 18 Mar Ecol Prog Ser l Feeding ecology of short-tailed shearwaters: breeding in Tasmania and foraging in the Antarctic? Henri Weimerskirch*, Yves Cherel CEBC - CNRS, F-79360 Beauvoir, France ABSTRACT- The food, feedlng and physiological ecology of foraging were studied in the short-tailed shearwater Puffinus tenujrostris of Tasmania, to establish whether this species can rely on Antarctic food to fledge its chick. Parents were found to use a 2-fold foraging strategy, on average performing 2 successive short trips at sea of 1 to 2 d duration followed by 1 long trip of 9 to 17 d. These long foraging tnps are the longest yet recorded for any seabird. During short trips the parents tend to lose mass, feed- ing the chick with Australian krill and fish larvae caught in coastal and neritic waters around Tasma- nia. The prey are caught at maximum diving depths of 13 m on average (maximum 30 m).During long trips, adults gain mass and feed their chlcks with a very rich mixture of stomach oil and digested food composed of a high diversity of prey including myctophid fish, sub-Antarctic krill and squids. Prey are probably caught mainly in the Polar Frontal Zone, at least 1000 km south of Tasmania, at maximum depths of 58 m on average (maximum 71 m). Long foraging trips in distant southern waters gave at least twice the yield of trips in close waters but during the former, yield decreased with the time spent for- aging, as indicated by the inverse relationship between time spent forag~ngand adult body condition. -

Large Pelagic Seabirds: Picture of Bird
Large Pelagic seabirds: Picture Albatrosses, Mollymawks & Giant Petrels of bird For idenDficaon and species info refer to: www.nzbirdsonline.org.nz Introduc4on Ecology and life history New Zealand has the highest diversity of Normal adult weight range: Adult Buller’s mollymawks can weigh as albatrosses and mollymawks with 12 liNle as 2.5kg while the Southern Royal Albatross can weigh up to species that breed on NZ's sub-antarcDc 10kg. Due to the variability of normal weight ranges between species islands and 7 endemic species. It is unlikely and within species it is recommended to calculate doses based on that large numbers of these birds would be individual body weights. effected during a single oil spill event Moult: Gradual, mostly during their non-breeding year but conDnues unless it occurs near a breeding colony. into breeding. Biennial wing moult - outer primaries one year, inner Although albatrosses are in the group primaries the next year. commonly called "tubenoses", they differ Breeding: All albatross species and the grey-headed mollymawk from other tubenose families in that their produce a single young every two years. Incubang and rearing a chick tube-shaped nostrils are separated and takes 1 year and then take one year to recover. located on either side of the bill. All birds The other mollymawk species and the two giant petrel species breed in the order Procellariformes (including once a year, usually from August to May. petrels and shearwaters) have three front- Lifespan: Long-lived facing toes with webbing. Diet: Water surface scavengers Personal protecve equipment (PPE): Appropriate PPE must be worn when capturing and handling oiled wildlife to prevent exposure to oil (disposable nitrile gloves, safety glasses/goggles, protecDon for clothing e.g. -

The Incidence, Functions and Ecological Significance of Petrel Stomach Oils
84 PROCEEDINGS OF THE NEW ZEALAND ECOLOGICAL SOCIETY, VOL. 24, 1977 THE INCIDENCE, FUNCTIONS AND ECOLOGICAL SIGNIFICANCE OF PETREL STOMACH OILS JOHN WARHAM Department of Zoology, University of Canterbury, Chrb;tchurch SUMMARY: Recent research into the origins and compositions of the stomach oils unique to sea~birds of the order Procellariifonnes is reviewed. The sources of these oils, most of which contain mainly wax esters and/or trigIycerides, is discussed in relation to the presence of such compounds in the marine environment. A number of functions are proposed as the ecological roles of the oils, including their use as slowIy~mobilisable energy and water reserves for adults and chicks and as defensive weaponry for surface-nesting species. Suggestions are made for further research, particularly into physiological and nutritional aspects. INTRODUCTION their table, a balm for their wounds, and a medicine Birds of the Order Procellariiforrnes (albatrosses, for their distempers." In New Zealand, Travers and fuJmars, shearwaters and other petrels) are peculiar Travers (1873) described how the Chatham Island in being able to store oil in their large, glandular and Morioris held young petrels over their mouths and very distensible fore-guts or proventriculi.. All petrel allowed the oil to drain directly into them. In some spedes so far examined, with the significant excep- years the St Kildans exported part of thei.r oil har~ tion of the diving petrels, Fam. Pelecanoididae, have vest, as the Australian mutton-birders stilI do with been found to contain oil at various times. The oi.1 oil from the chicks of P. tenuirostris. This has been occurs in both adults and chicks, in breeders and used as a basis for sun~tan lotions, but most nowa- non~breeders, and in birds taken at sea and on land. -

Intra- and Inter-Annual Breeding Season Diet of Leach's Storm-Petrel (Oceanodroma Leucorhoa) at a Colony in Southern Oregon
INTRA- AND INTER-ANNUAL BREEDING SEASON DIET OF LEACH'S STORM-PETREL (OCEANODROMA LEUCORHOA) AT A COLONY IN SOUTHERN OREGON by MICHELLE ANDRIESE SCHUITEMAN A THESIS Presented to the Department ofBiology and the Graduate School ofthe University ofOregon in partial fulfillment ofthe requirements for the degree of Master ofScience December 2006 11 "Intra- and Inter-annual Breeding Season Diet ofLeach's Storm-petrel (Oceanodroma leucorhoa) at a Colony in Southern Oregon" a thesis prepared by Michelle Andriese Schuiteman in partial fulfillment ofthe requirments for the Master ofScience degree in the Department ofBiology. This thesis has been approved and accepted by: Date Committee in Charge: Dr Alan Shanks, Chair Dr. Jan Hodder Dr. William Sydeman Accepted by: Dean ofthe Graduate School 111 © 2006 Michelle Schuiteman lV An Abstract ofthe Thesis of Michelle Andriese Schuiteman for the degree of Master ofScience in the Department ofBiology to be taken December 2006 Title: INTRA- AND INTER-ANNUAL BREEDING SEASON DIET OF LEACH'S STORM-PETREL (OCEANODROMA LEUCORHOA) AT A COLONY IN SOUTHERN OREGON The oceanic habitat varies on multiple spatial and temporal scales. Aspects ofthe ecology oforganisms that utilize this habitat can, in certain cases, be used as indicators of ocean conditions. In this study, diet ofthe Leach's storm-petrel (Oceanodroma leucorhoa) is examined to determine ifevidence ofchanging ocean conditions can be found in the diet. Regurgitations were collected from the birds in order to describe diet. Euphausiids and fish composed 80 - 90% ofthe diet in both years, with composition of each diametrically different between years. Other items found in samples included . hyperiid and gammariid amphipods, cephalopods, plastic pieces and a new species of Cirolanid isopod. -

SYN Seabird Curricul
Seabirds 2017 Pribilof School District Auk Ecological Oregon State Seabird Youth Network Pribilof School District Ram Papish Consulting University National Park Service Thalassa US Fish and Wildlife Service Oikonos NORTAC PB i www.seabirdyouth.org Elementary/Middle School Curriculum Table of Contents INTRODUCTION . 1 CURRICULUM OVERVIEW . 3 LESSON ONE Seabird Basics . 6 Activity 1.1 Seabird Characteristics . 12 Activity 1.2 Seabird Groups . 20 Activity 1.3 Seabirds of the Pribilofs . 24 Activity 1.4 Seabird Fact Sheet . 26 LESSON TWO Seabird Feeding . 31 Worksheet 2.1 Seabird Feeding . 40 Worksheet 2.2 Catching Food . 42 Worksheet 2.3 Chick Feeding . 44 Worksheet 2.4 Puffin Chick Feeding . 46 LESSON THREE Seabird Breeding . 50 Worksheet 3.1 Seabird Nesting Habitats . .5 . 9 LESSON FOUR Seabird Conservation . 63 Worksheet 4.1 Rat Maze . 72 Worksheet 4.2 Northern Fulmar Threats . 74 Worksheet 4.3 Northern Fulmars and Bycatch . 76 Worksheet 4.4 Northern Fulmars Habitat and Fishing . 78 LESSON FIVE Seabird Cultural Importance . 80 Activity 5.1 Seabird Cultural Importance . 87 LESSON SIX Seabird Research Tools and Methods . 88 Activity 6.1 Seabird Measuring . 102 Activity 6.2 Seabird Monitoring . 108 LESSON SEVEN Seabirds as Marine Indicators . 113 APPENDIX I Glossary . 119 APPENDIX II Educational Standards . 121 APPENDIX III Resources . 123 APPENDIX IV Science Fair Project Ideas . 130 ii www.seabirdyouth.org 1 INTRODUCTION 2017 Seabirds SEABIRDS A seabird is a bird that spends most of its life at sea. Despite a diversity of species, seabirds share similar characteristics. They are all adapted for a life at sea and they all must come to land to lay their eggs and raise their chicks. -

Mollymawk Chick Numbers and Survival on Campbell Island, November 2019–March 2020
Mollymawk Chick Numbers and Survival on Campbell Island, November 2019–March 2020 Peter G.H. Frost 1 and Claudia Mischler 2 1 Science Support Service, 87 Ikitara Road, Whanganui 4500 [email protected] 2 Department of Conservation, 15 Wairepo Road, Twizel 7901 [email protected] Summary An aerial photographic survey of the mixed Campbell Mollymawk, Thalassarche impavida, and Grey-headed Mollymawk, Thalassarche chrysostoma, colonies along the north-east coast of Campbell Island was carried out on 17 March 2020. At this time, most of the birds present at these colonies would have been chicks. The aim of the study was therefore to establish the number of chicks in these colonies and relate this to the number of nesting adults estimated from a combined aerial and ground- level photographic survey carried out earlier in the nesting season, 18–24 November 2019. Campbell Mollymawks made 90 % of these nesting birds, with Grey-headed Mollymawks the remainder. Counts were made independently by two analysts and the results averaged. A total of 7890 chicks of both species combined were recorded across four colony groups: Bull Rock South (containing 54.6 % of the total); Bull Rock North (24.8 %); the Eastern colonies (19.1 %); and Sorensen Tarn (1.5 %). Assessed against the number of apparently active nests in November 2019 (i.e. ones assumed to contain an egg, taken to be 82 % of the apparently occupied nests counted at that time), the survival rate from November to March at these colonies was: Bull Rock South, 60.6 %; Bull Rock North, 62.6 %; the Eastern colonies, 70.4 %; and Sorensen Tarn, 99.1 %. -

Diet and Trophic Position of Leach's Storm-Petrel Oceanodroma
CORE Metadata, citation and similar papers at core.ac.uk Provided by Memorial University Research Repository MARINE ECOLOGY PROGRESS SERIES Vol. 322: 291–301, 2006 Published September 20 Mar Ecol Prog Ser Diet and trophic position of Leach’s storm-petrel Oceanodroma leucorhoa during breeding and moult, inferred from stable isotope analysis of feathers April Hedd*, William A. Montevecchi Cognitive and Behavioural Ecology Programme, Departments of Psychology and Biology, Memorial University of Newfoundland, St. John’s, Newfoundland A1B 3X9, Canada ABSTRACT: We combined conventional dietary sampling (2002) with stable isotope analysis of nitro- gen (δ15N) and carbon (δ13C) in feathers of Leach’s storm-petrels Oceanodroma leucorhoa (2001 and 2002) to investigate temporal, sex- and age-related variation in diet and trophic position, at Baccalieu Island, Newfoundland, Canada. Nestlings are fed mainly fish and crustaceans (ca. 90 and 9% by mass, respectively) toward the end of chick-rearing. The bulk of the fish were vertically migrating myctophids, associated with the offshore environment. Crustaceans were mainly parasitic hyperiid amphipods Hyperia galba, commonly found nearshore in association with scyphozoan jellyfish hosts. δ15N values in summer-grown feathers were similar between years, but differed between age-classes in 2001 when chicks occupied slightly higher trophic level (3.2 vs. 3.1). δ13C values in summer-grown feathers were lower for chicks than adults. Predictions from a 3-source dual-isotope linear mixing model indicated that adults consumed 47% glacier lanternfish Benthosema glaciale, 5% capelin Mal- lotus villosis and 49% H. galba in 2001, while in 2002 respective values were 87, 6 and 7%. -

A Story About Albatross
A Story About Albatross Tracking their Travels and Tracking Plastic Trash © Sophie Webb 2004 This is a story about tracking albatross and tracking plastic trash. In particular, this story is about the dark albatross (right side of image) – this is the Black-footed albatross. These birds criss-cross the entire Pacific Ocean like you and I criss-cross our backyards! 1 “If we didn’t clean our shorelines, where could the litter go?” “How can your coastal clean- up efforts benefit these unique birds?” These are two important questions to think about during this presentation. 2 Seabird Diversity H. Nevins J.Harvey Alcid WWW.nzbirds.com Penguin Pelican Petrel H. Nevins Four main orders of seabirds: Sphenisciformes - Penguins Procellariiformes – Albatrosses, Shearwaters, Fulmars, & Petrels Pelecaniformes - Pelicans, Cormorants, Boobies, Frigate birds Charadriiformes - Gulls, Terns, & Alcids There are 4 main orders of seabirds: Sphenisciformes, Procellariiformes, Pelecaniformes, and Charadriiformes. Seabirds that belong to the order Procellariiformes are among the most pelagic and far-ranging of seabirds that occur in all the oceans. This story is about seabirds from this group. 3 Seabird Feeding Methods FEEDERS Plunging (Ashmole 1971) Seabirds feed in three main ways: (1) they collect food items from the surface, (2) they plunge to capture submerged prey, and (3) they use their wings and feet to fly or swim underwater. The foraging methods of seabirds influence their ability to gather different types of prey and marine debris (e.g., floating versus sinking). Whereas most plastics float on the surface, other types may float deeper down in the water column. Thus, not all the debris that sinks at the surface ends up in the bottom of the sea. -
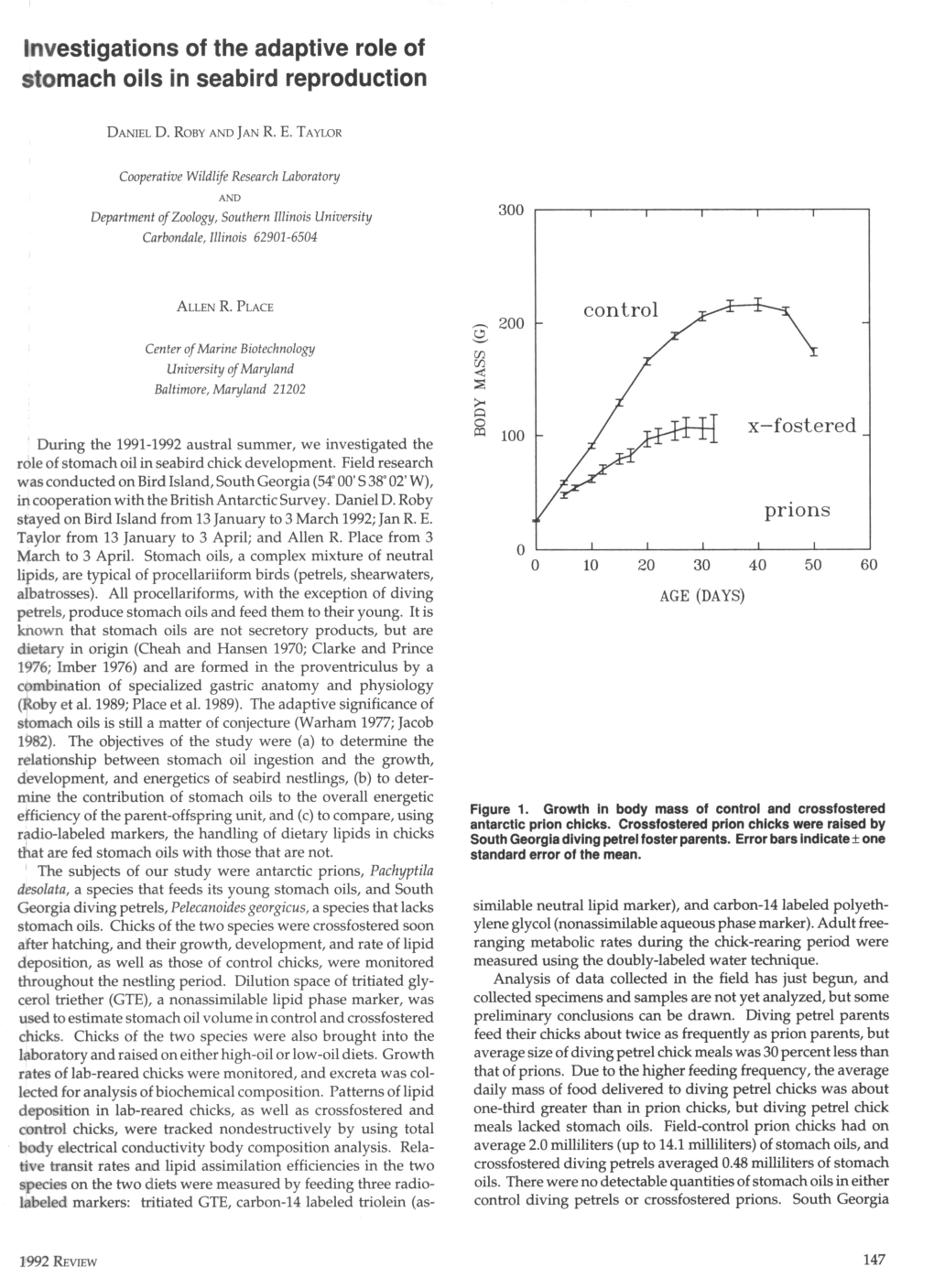
Physiological Aspects of Stomach-Oil Formation in Antarctic Seabirds DANIEL D
Physiological aspects of stomach-oil formation in antarctic seabirds DANIEL D. ROBY and JAN R.E. TAYLOR, Alaska Cooperative Fish and Wildlife Research Unit, University ofAlaska, Fairbanks, Alaska 99775-0990 ALLEN R. PLACE, Center of Marine Biotechnology, Maryland Technological Institute, Baltimore, Maryland 21202 uring the 1991-1992 austral summer, we conducted feeds its young stomach oils, and South Georgia diving petrels D fieldwork on Bird Island (54°00S 38°02W), South Geor- (Pelecanoides georgicus), a species that lacks stomach oils. gia, in cooperation with the British Antarctic Survey. This Chicks of the two species were brought into the laborato- research project was designed to investigate the role of stom- ry and raised on either high-oil or low-oil diets. The low-oil ach oils in digestive physiology, development, and reproduc- diet consisted of homogenized hill (Euphausia superba) and tive energetics of seabirds. Samples and specimens that were stomach oil [collected from giant petrel (Macronectes haul) collected in the field were analyzed in the laboratory during chicks] in a ratio (weight-to-weight) of 22:1; whereas the high- 1992-1993. oil diet consisted of homogenized bill and stomach oil in a Stomach oils, a complex mixture of neutral dietary lipids, ratio of 14:5. Chicks were fed one meal each day in the lab for are typical of procellariiform birds (petrels, shearwaters, alba- 6 days. Growth rates were monitored and all excreta was col- trosses). All Procellariiformes, with the exception of diving lected during this period. Average daily mass increments of petrels (Pelecanoides spp.) produce stomach oils and feed lab-reared chicks were similar to or greater than those of them to their young (Clarke and Prince 1976; Warham 1977). -

Foraging Strategy of Wandering Albatrosses Through the Breeding Season: a Study Using Satellite Telemetry
The Auk 110(2):325-342, 1993 FORAGING STRATEGY OF WANDERING ALBATROSSES THROUGH THE BREEDING SEASON: A STUDY USING SATELLITE TELEMETRY HENRI WEIMERSKIRCH, MARC SALAMOLARD, FRANCOISSARRAZIN, AND PIERREJOUVENTIN Centred'Etudes Biologiques des Animaux Sauvages, Centre National de la RechercheScientifique, 79360 Beauvoir, France ABSTRACT.--Satellitetelemetry of Wandering Albatrosses(Diomedea exulans) breeding on the Crozet Islands, southwesternIndian Ocean, revealed two distinct foraging strategies during successivestages of the breedingseason: systematic foraging over extensivedistances; and useof specificareas close to the colony.During early incubation,Wandering Albatrosses foragedover pelagicwaters at an averagerange of 1,284kin. The length of the foraging trips decreasedtowards the end of the incubation period. During the first month of chick rearing when parentsbrood alternately for short periods,the foraging range, distancecovered, and area prospectedwere further reduced.Males tended to return to an individual foraging area, locatedat the edge of the continental shelf, that had previouslybeen visited during the long trips of the incubation period. Femalesmostly prospectedpelagic waters just off the shelf. After the chick had been left alone on the nest,birds exhibited a two-fold strategy,combining long foraging trips over pelagic waters with short trips over the shelf. Generally, both sexes headed for and foraged over an extensivepelagic sector.Some males also foraged over the Kerguelen shelf. Females tended to forage over more northerly waters than males. The duration of the foraging trips was most closelyrelated to the total distancecovered, but also to the maximumrange during the long trips of the chick-rearingperiod and to a lesserextent during the incubation period. There were no such significantrelationships in the caseof short trips.