Space Available for Trans-Sacral Implants to Treat Fractures of The
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Lab #23 Anal Triangle
THE BONY PELVIS AND ANAL TRIANGLE (Grant's Dissector [16th Ed.] pp. 141-145) TODAY’S GOALS: 1. Identify relevant bony features/landmarks on skeletal materials or pelvic models. 2. Identify the sacrotuberous and sacrospinous ligaments. 3. Describe the organization and divisions of the perineum into two triangles: anal triangle and urogenital triangle 4. Dissect the ischiorectal (ischioanal) fossa and define its boundaries. 5. Identify the inferior rectal nerve and artery, the pudendal (Alcock’s) canal and the external anal sphincter. DISSECTION NOTES: The perineum is the diamond-shaped area between the upper thighs and below the inferior pelvic aperture and pelvic diaphragm. It is divided anatomically into 2 triangles: the anal triangle and the urogenital (UG) triangle (Dissector p. 142, Fig. 5.2). The anal triangle is bounded by the tip of the coccyx, sacrotuberous ligaments, and a line connecting the right and left ischial tuberosities. It contains the anal canal, which pierced the levator ani muscle portion of the pelvic diaphragm. The urogenital triangle is bounded by the ischiopubic rami to the inferior surface of the pubic symphysis and a line connecting the right and left ischial tuberosities. This triangular space contains the urogenital (UG) diaphragm that transmits the urethra (in male) and urethra and vagina (in female). A. Anal Triangle Turn the cadaver into the prone position. Make skin incisions as on page 144, Fig. 5.4 of the Dissector. Reflect skin and superficial fascia of the gluteal region in one flap to expose the large gluteus maximus muscle. This muscle has proximal attachments to the posteromedial surface of the ilium, posterior surfaces of the sacrum and coccyx, and the sacrotuberous ligament. -

Surgical Approaches to Fractures of the Acetabulum and Pelvis Joel M
Surgical Approaches to Fractures of the Acetabulum and Pelvis Joel M. Matta, M.D. Sponsored by Mizuho OSI APPROACHES TO THE The table will also stably position the ACETABULUM limb in a number of different positions. No one surgical approach is applicable for all acetabulum fractures. KOCHER-LANGENBECK After examination of the plain films as well as the CT scan the surgeon should APPROACH be knowledgeable of the precise anatomy of the fracture he or she is The Kocher-Langenbeck approach is dealing with. A surgical approach will primarily an approach to the posterior be selected with the expectation that column of the Acetabulum. There is the entire reduction and fixation can excellent exposure of the be performed through the surgical retroacetabular surface from the approach. A precise knowledge of the ischial tuberosity to the inferior portion capabilities of each surgical approach of the iliac wing. The quadrilateral is also necessary. In order to maximize surface is accessible by palpation the capabilities of each surgical through the greater or lesser sciatic approach it is advantageous to operate notch. A less effective though often the patient on the PROfx® Pelvic very useful approach to the anterior Reconstruction Orthopedic Fracture column is available by manipulation Table which can apply traction in a through the greater sciatic notch or by distal and/or lateral direction during intra-articular manipulation through the operation. the Acetabulum (Figure 1). Figure 2. Fractures operated through the Kocher-Langenbeck approach. Figure 3. Positioning of the patient on the PROfx® surgical table for operations through the Kocher-Lagenbeck approach. -

The Pelvis Structure the Pelvic Region Is the Lower Part of the Trunk
The pelvis Structure The pelvic region is the lower part of the trunk, between the abdomen and the thighs. It includes several structures: the bony pelvis (or pelvic skeleton) is the skeleton embedded in the pelvic region of the trunk, subdivided into: the pelvic girdle (i.e., the two hip bones, which are part of the appendicular skeleton), which connects the spine to the lower limbs, and the pelvic region of the spine (i.e., sacrum, and coccyx, which are part of the axial skeleton) the pelvic cavity, is defined as the whole space enclosed by the pelvic skeleton, subdivided into: the greater (or false) pelvis, above the pelvic brim , the lesser (or true) pelvis, below the pelvic brim delimited inferiorly by the pelvic floor(or pelvic diaphragm), which is composed of muscle fibers of the levator ani, the coccygeus muscle, and associated connective tissue which span the area underneath the pelvis. Pelvic floor separate the pelvic cavity above from the perineum below. The pelvic skeleton is formed posteriorly (in the area of the back), by the sacrum and the coccyx and laterally and anteriorly (forward and to the sides), by a pair of hip bones. Each hip bone consists of 3 sections, ilium, ischium, and pubis. During childhood, these sections are separate bones, joined by the triradiate hyaline cartilage. They join each other in a Y-shaped portion of cartilage in the acetabulum. By the end of puberty the three bones will have fused together, and by the age of 25 they will have ossified. The two hip bones join each other at the pubic symphysis. -

The Stoppa Combined with Iliac Fossa Approach for the Treatment of Both-Column Acetabular Fractures Yun Yang†, Chang Zou† and Yue Fang*
Yang et al. Journal of Orthopaedic Surgery and Research (2020) 15:588 https://doi.org/10.1186/s13018-020-02133-3 RESEARCH ARTICLE Open Access The Stoppa combined with iliac fossa approach for the treatment of both-column acetabular fractures Yun Yang†, Chang Zou† and Yue Fang* Abstract Background: At present, the choice of surgical approach for both-column fractures is still controversial. The purpose of this study was to explore the efficacy of the Stoppa combined with iliac fossa (S+IF) approach in the treatment of both-column fractures. Methods: In this retrospective case series, 76 patients were included in the study from 2014 to 2018. They were divided into two groups according to the surgical approaches. The differences of intraoperative blood loss, operative time, quality of reduction, clinical outcome, and perioperative complications were compared between the two groups. Results: All patients had undergone the IL approach or the S+IF approach. The average operative time was 156.2 min (110~210 min) in group I and 126.5 min (80~180 min) in group II (P < 0.001). The average blood loss in group I was 784.1 ml, while the average blood loss in group II was 625.3 ml (P = 0.007). According to Matta’s criteria, 28 cases obtained anatomic reduction and 12 cases got imperfect reduction in group I; 21 cases obtained anatomic reduction and 7 cases got imperfect reduction in group II (P > 0.05). The clinical outcome (excellent to good) was 66% in group I versus 69% in group II (P > 0.05). -

7-Pelvis Nd Sacrum.Pdf
Color Code Important PELVIS & SACRUM Doctors Notes Notes/Extra explanation EDITING FILE Objectives: Describe the bony structures of the pelvis. Describe in detail the hip bone, the sacrum, and the coccyx. Describe the boundaries of the pelvic inlet and outlet. Identify the articulations of the bony pelvis. List the major differences between the male and female pelvis. List the different types of female pelvis. Overview: • check this video to have a good picture about the lecture: https://www.youtube.com/watch?v=PJOT1cQHFqA https://www.youtube.com/watch?v=3v5AsAESg1Q&feature=youtu.be • BONY PELVIS = 2 Hip Bones (lateral) + Sacrum (Posterior) + Coccyx (Posterior). • Hip bone is composed of 3 parts = Superior part (Ilium) + Lower anterior part (Pubis) + Lower posterior part (Ischium) only on the boys slides’ BONY PELVIS Location SHAPE Structure: Pelvis can be regarded as a basin with holes in its walls. The structure of the basin is composed of: Pelvis is the region of the Bowl shaped 4 bones 4 joints trunk that lies below the abdomen. 1-sacrum A. Two hip bones: These form the lateral and 2-ilium anterior walls of the bony pelvis. 3-ischium B. Sacrum: It forms most of the posterior wall. 4-pubic C. Coccyx: It forms most of the posterior wall. 5-pubic symphysis 6-Acetabulum Function # Primary: The skeleton of the pelvis is a basin-shaped ring of bones with holes in its wall connecting the vertebral column to both femora. Its primary functions are: bear the weight of the upper body when sitting and standing; transfer that weight from the axial skeleton to the lower appendicular skeleton when standing and walking; provide attachments for and withstand the forces of the powerful muscles of locomotion and posture. -

Study of Significance of Total Pelvic Height and Pelvic Width in Sex Determination of Human Innominate Bone in Gujarat Region Sudarshan Gupta*, Kiran Arora **
Original Article GCSMC J Med Sci Vol (II) No (II) July-December 2013 Study of Significance of Total Pelvic Height and Pelvic Width in Sex Determination of Human Innominate Bone in Gujarat Region Sudarshan Gupta*, Kiran Arora ** Abstract : Introduction : Sex determination of the unknown hip bone either of whole skeleton or any part of it, is always a field of research not only for anatomist but also for forensic expert, anthropologist and archeologist. Hip bone was considered as ideal bone for sex determination after skull.Material and Method : This study was carried out on 100 dried human innominate bones out of them 40 were female and 60 were male bones. Gross morphometric parameters like total pelvic height, pelvic width was measured and ratio of pelvic width and total pelvic height was calculated.Results : Mean value of total pelvic height was higher in male(193.85 mm) compared to female(179.45mm), mean value of pelvic width was also higher in male (137.31 mm ) than female ( 133.24mm). It was found that mean value of ratio between pelvic width and total pelvic height was higher in female (0.74) as compared to male (0.70). The same was found statistically highly significant (P value <0.0001).Conclusion and Recommendation : The finding of this study showed that there were statistically significant gender difference were present in gross morphometric parameters, hence these measurements of the hip bone can be used for sex determination of unknown skeletons and in the forensic science for medicolegal cases. Key Words : Total pelvic height, pelvic width, innominate bone Introduction : total pelvic height, pelvic width, acetabular diameter, pubic Innominate bone is also known as hip bone. -
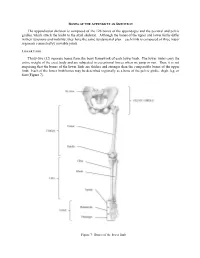
Bones of the Appendicular Skeleton
BONES OF THE APPENDICULAR SKELETON The appendicular skeleton is composed of the 126 bones of the appendages and the pectoral and pelvic girdles, which attach the limbs to the axial skeleton. Although the bones of the upper and lower limbs differ in their functions and mobility, they have the same fundamental plan – each limb is composed of three major segments connected by movable joints. LOWER LIMB Thirty-two (32) separate bones form the bony framework of each lower limb. The lower limbs carry the entire weight of the erect body and are subjected to exceptional forces when we jump or run. Thus, it is not surprising that the bones of the lower limb are thicker and stronger than the comparable bones of the upper limb. Each of the lower limb bones may be described regionally as a bone of the pelvic girdle, thigh, leg, or foot (Figure 7). Figure 7: Bones of the lower limb Pelvic (Hip) Girdle (Marieb / Hoehn – Chapter 7; Pgs. 234 – 238) The pelvic girdle is formed by the paired os coxae (coxal bones). Together with the sacrum and coccyx of the axial skeleton, this group of bones forms the bony pelvis. The ability to bear weight is more important in the pelvic girdle than the pectoral girdle. Thus, the os coxae are heavy and massive with a firm attachment to the axial skeleton. Each os coxa is a result of the fusion of three bones: the ilium, ischium, and pubis. These three bones fuse at the deep hemispherical socket, the acetabulum, which receives the femur. Figure 8: Right os coxa, lateral and medial views A. -

The Appendicular Skeleton
Unit 2 Lecture 6 Unit 2 Lecture 6 THE APPENDICULAR SKELETON The APPENDICULAR SKELETON consists of the pectoral and pelvic girdles and the upper and lower extremities. The PECTORAL (SHOULDER) GIRDLE attaches the bones of the upper extremities to the axial skeleton and supports the shoulder. The Clavicle serves as a brace to keep the arm away from the thorax. It has two curves (one convex, one concave); juncture of these curves is where most fractures to the clavicle occur. The Scapula (shoulder blade) are broad triangular bones with spines, acromium processes (articulates with the clavicle), bodies, coracoid processes, glenoid cavities (fossa) receives the head of the humerus. They provide many points for muscle attachment. The UPPER EXTREMITY consists of 60 bones (thirty per limb) that provide the framework for muscle attachment and functions in levers that move the limb and its parts. The humerus is the longest bone in the upper extremity. It has a head (proximal end), anatomical neck (former site of the epiphyseal plate), greater and lesser tubercle and intertubercular sulcus, deltoid tuberosity (point of attachment for deltoid muscle), a capitulum (articulates with the head of the radius) radial fossa (receives the head of the radius when forearm is bent), a trochlea: looks like a spool (articulates with the ulna), and a coronoid fossa (receives part of the ulna when forearm is bent), body (main shaft), olecranon fossa (receives olecranon when forearm is extended), medial (“funny bone”) and lateral epicondyles (point of muscle attachment). The surgical neck is the site of most fractures. The ulna and is longer than the radius and overlaps the humerus posteriorly. -
Diagnosis of Inflammatory Disease in the Acute Abdomen
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1939 Diagnosis of inflammatory disease in the acute abdomen Morris A. Sonderegger University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Sonderegger, Morris A., "Diagnosis of inflammatory disease in the acute abdomen" (1939). MD Theses. 777. https://digitalcommons.unmc.edu/mdtheses/777 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. DIAGNOSIS OF INFLAMMATORY DISEASE IN THE ACUTE ABDOMEN BY MOBRIS A. SONDEREGGER SENIOR THESIS , I PRESENTED TO i'HE COLLEGE OF MEDI CINE I ; UNIVERSITY OF NEBRASKA OUAHA. 1939 ,-., . l fl81068 TABLE OF CONTENTS INTRODUCTION•••••••••••••••••••••••••••••••••••••••l MECHANISM OF PAIN •••••••••••• • •• • •• • •• • •• ••.••••• .·.2 ACUTE APPENDICITIS•••••••••••••••••••••••••••••••••7 DIFFERENTIAL DIAGNOSIS OF APPENDICITIS••••••••••••44 CHOLECYSTITIS ••••••••••••••••••••••••••••••••••••• 58 DIFFERENTIAL DIAGNOSIS OF CHOLEOYSTITIS ••••••••••• 73 ACUTE PERITONITIS •• •••••••••••••••••••••••••••••••82 DIFFERENTIAL DIAGNOSIS OF PERITONITIS.••.•• •• •• •• 104 -

Appendicular Skeleton
APPENDICULAR SKELETON I. Shoulder girdle A. Clavicle 1. Sternal end 2. Acromial end 3. Conoid tubercle B. Scapula 1. Spine 2. Acromion 3. Coracoid process 4. Glenoid fossa 5. Superior angle 6. Inferior angle 7. Superior border 8. Vertebral(Medial) border 9. Axillary (Lateral) border 10. Supraspinatus (Supraspinous) fossa 11. Infraspinatus (Infraspinous) fossa 12. Costal surface 13. Subscapular fossa 14. Scapular notch 15. Root (Base) Scapular Spine II. Upper Extremity A. Humerus 1. Head 2. Neck 3. Greater tubercle i. Superior facet ii. Middle facet iii. Inferior facet 4. Lesser tubercle 5. Intertubercular groove (sulcus) 6. Body 7. Deltoid tubercle 8. Coronoid fossa 9. Olecranon fossa 10. Trochlea (Medial condyle) 11. Capitulum (Lateral condyle) 12. Medial epicondyle 13. Lateral epicondyle 14. Medial Supracondylar ridge 15. Lateral Supracondylar ridge 16. Radial fossa B. Ulna 1. Head 2. Neck 3. Olecranon process 4. Semilunar (trochlear) notch 5. Coronoid process 6. Radial notch 7. Styloid process 8. Supinator crest 9. Ulnar tuberosity C. Radius 1. Head 2. Neck 3. Radial tuberosity 4. Styloid process 5. Ulnar Notch D. Carpals 1. Scaphoid 2. Lunate 3. Triquetrum (Triquetral) 4. Pisiform 5. Trapezium 6. Trapezoid 7. Capitate 8. Hamate a. Hook of Hamate E. Metacarpals 1. Head 2. Shaft 3. Base F. Phalanges 1. Base 2. Shaft 3. Head 4. Thumb (Pollex) a. Proximal b. Distal 5. Fingers (II-V) a. Proximal b. Middle c. Distal III. Pelvis A. Sacrum B. Coccyx C. Coxal (Hip) bones 1. Ilium a. Iliac crest b. Anterior superior iliac spine (ASIS) c. Anterior inferior iliac spine (AIIS) d. Posterior superior iliac spine (PSIS) e. -
Surgical Approaches to Fractures of the Acetabulum and Pelvis
Surgical Approaches to Fractures of the Acetabulum and Pelvis Joel M. Matta, M.D. Sponsored by Surgical Approaches to Fractures of the Acetabulum and Pelvis APPROACHES TO THE The table will also stably position the limb in a number of different positions. ACETABULUM No one surgical approach is applicable KOCHER-LANGENBECK for all acetabulum fractures. After APPROACH examination of the plain films as well as the CT scan the surgeon should be knowledgeable of the precise anatomy The Kocher-Langenbeck approach is of the fracture he or she is dealing with. primarily an approach to the posterior A surgical approach will be selected column of the Acetabulum. There is with the expectation that the entire excellent exposure of the reduction and fixation can be retroacetabular surface from the ischial performed through the surgical tuberosity to the inferior portion of the approach. A precise knowledge of the iliac wing. The quadrilateral surface is capabilities of each surgical approach is accessible by palpation through the also necessary. In order to maximize greater or lesser sciatic notch. A less the capabilities of each surgical effective though often very useful approach it is advantageous to operate approach to the anterior column is the patient on the PROfx® Pelvic available by manipulation through the Reconstruction Orthopedic Fracture greater sciatic notch or by intra- Table which can apply traction in a articular manipulation through the distal and/or lateral direction during Acetabulum (Figure 1). the operation. Mizuho OSI 2018 1 NW0709 Rev C Surgical Approaches to Fractures of the Acetabulum and Pelvis Figure 2. Fractures operated through the Kocher-Langenbeck approach. -

Of the Ilium Iliac Fossa Took Bone in the Application of Autologous Bone Graft
International Surgery Journal Gao QK et al. Int Surg J. 2015 May;2(2):218-220 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: 10.5455/2349-2902.isj20150518 Research Article “Open window” of the ilium iliac fossa took bone in the application of autologous bone graft Qian Kun Gao, Wei Li, Min Dai* The First Affiliated Hospital of Nanchang University, Nanchang, China Received: 05 February 2015 Revised: 17 February 2015 Accepted: 22 March 2015 *Correspondence: Dr. Min Dai, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Objective: To explore practical, simple, and less complications method of iliac cut. Methods: A retrospective analysis of 23 patients with iliac bone taken between the June 2011 - June 2013. Using “windowed iliac fossa” to cut single or double cortex ilium, then count donor site complications and related factors. Results: There is no abnormal appearance at the taken sit of the iliac, also there is no local pain or tenderness touch. The skin feeling of hip and thigh are normal, the VAS is 7-9, the department of belt are also unaffected. Conclusions: As an improved method of bone taken in the autogenous bone graft, the complications of “windowed iliac fossa” decreased significantly than the traditional method, as a new methods of iliac cut, which is worth popularizing in clinical practice.