Terra Australis 37
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hatching Plasticity in the Tropical Gastropod Nerita Scabricosta
Invertebrate Biology x(x): 1–10. Published 2016. This article is a U.S. Government work and is in the public domain in the USA. DOI: 10.1111/ivb.12119 Hatching plasticity in the tropical gastropod Nerita scabricosta Rachel Collin,a Karah Erin Roof, and Abby Spangler Smithsonian Tropical Research Institute, 0843-03092 Balboa, Panama Abstract. Hatching plasticity has been documented in diverse terrestrial and freshwater taxa, but in few marine invertebrates. Anecdotal observations over the last 80 years have suggested that intertidal neritid snails may produce encapsulated embryos able to signifi- cantly delay hatching. The cause for delays and the cues that trigger hatching are unknown, but temperature, salinity, and wave action have been suggested to play a role. We followed individual egg capsules of Nerita scabricosta in 16 tide pools to document the variation in natural time to hatching and to determine if large delays in hatching occur in the field. Hatching occurred after about 30 d and varied significantly among tide pools in the field. Average time to hatching in each pool was not correlated with presence of potential preda- tors, temperature, salinity, or pool size. We also compared hatching time between egg cap- sules in the field to those kept in the laboratory at a constant temperature in motionless water, and to those kept in the laboratory with sudden daily water motion and temperature changes. There was no significant difference in the hatching rate between the two laboratory treatments, but capsules took, on average, twice as long to hatch in the laboratory as in the field. -

Of Penguins and Polar Bears Shapero Rare Books 93
OF PENGUINS AND POLAR BEARS Shapero Rare Books 93 OF PENGUINS AND POLAR BEARS EXPLORATION AT THE ENDS OF THE EARTH 32 Saint George Street London W1S 2EA +44 20 7493 0876 [email protected] shapero.com CONTENTS Antarctica 03 The Arctic 43 2 Shapero Rare Books ANTARCTIca Shapero Rare Books 3 1. AMUNDSEN, ROALD. The South Pole. An account of “Amundsen’s legendary dash to the Pole, which he reached the Norwegian Antarctic Expedition in the “Fram”, 1910-1912. before Scott’s ill-fated expedition by over a month. His John Murray, London, 1912. success over Scott was due to his highly disciplined dogsled teams, more accomplished skiers, a shorter distance to the A CORNERSTONE OF ANTARCTIC EXPLORATION; THE ACCOUNT OF THE Pole, better clothing and equipment, well planned supply FIRST EXPEDITION TO REACH THE SOUTH POLE. depots on the way, fortunate weather, and a modicum of luck”(Books on Ice). A handsomely produced book containing ten full-page photographic images not found in the Norwegian original, First English edition. 2 volumes, 8vo., xxxv, [i], 392; x, 449pp., 3 folding maps, folding plan, 138 photographic illustrations on 103 plates, original maroon and all full-page images being reproduced to a higher cloth gilt, vignettes to upper covers, top edges gilt, others uncut, usual fading standard. to spine flags, an excellent fresh example. Taurus 71; Rosove 9.A1; Books on Ice 7.1. £3,750 [ref: 96754] 4 Shapero Rare Books 2. [BELGIAN ANTARCTIC EXPEDITION]. Grande 3. BELLINGSHAUSEN, FABIAN G. VON. The Voyage of Fete Venitienne au Parc de 6 a 11 heurs du soir en faveur de Captain Bellingshausen to the Antarctic Seas 1819-1821. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Goose Barnacle
Fisheries Pêches and Oceans et Océans DFO Science Pacific Region Stock Status Report C6-06 (1998) Rostral - Carinal Length GOOSE BARNACLE Background The Fishery The goose barnacle (Pollicipes polymerus) ranges from southern Alaska to Baja California on the First Nations people have long used goose upper two-thirds of the intertidal zone on exposed or barnacles as food. Goose barnacles have semi-exposed rocky coasts. been commercially harvested sporadically since the 1970s, and continuously since Goose barnacles are hemaphrodidic (one individual has both sexes). They mature at 14-17 mm rostral- 1985. They are hand harvested with various carinal length or one to 3 years of age. Spawning is design cutting tools, and then stored and from late April to early October, with peak spawning shipped as live product. in July, producing 475,000 - 950,000 embryos/adult /season. Larvae are planktonic for 30-40 days, and Goose barnacles have long been recognized settle in suitable habitat at 0.5mm length. as a delicacy in Spain, Portugal and France. Growth is rapid the first year (11-15 mm rostral- The major market for Canadian west coast carinal length) and slows thereafter to 1-3 mm/yr. goose barnacles is Spain, particularly the Maximum size is 45 mm rostral-carinal length, 153 Barcelona area. The market price in Spain peduncle length. Maximum age is unknown. The varies with season and availability from other muscular stalk (peduncle) is analogous to the sources. muscular tail of shrimp, prawns or lobster. Harvesters use a modified cutting and prying tool to Accessibility to the wave swept areas of the free goose barnacles from their substrates and west coast of Vancouver Island (Statistical collect and sort them by hand. -

Marine Fish Conservation Global Evidence for the Effects of Selected Interventions
Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca0 K. Smith & William J. Sutherland CONSERVATION EVIDENCE SERIES SYNOPSES Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca K. Smith and William J. Sutherland Conservation Evidence Series Synopses 1 Copyright © 2021 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Taylor, N., Clarke, L.J., Alliji, K., Barrett, C., McIntyre, R., Smith, R.K., and Sutherland, W.J. (2021) Marine Fish Conservation: Global Evidence for the Effects of Selected Interventions. Synopses of Conservation Evidence Series. University of Cambridge, Cambridge, UK. Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Circling fish in the waters of the Halmahera Sea (Pacific Ocean) off the Raja Ampat Islands, Indonesia, by Leslie Burkhalter. Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ -

Five Nations Multi-Species Fishery Management Plan, April 1, 2021
PACIFIC REGION FIVE NATIONS MULTI-SPECIES FISHERY MANAGEMENT PLAN April 1, 2021 – March 31, 2022 SALMON, GROUNDFISH, CRAB, PRAWN, GOOSENECK BARNACLE, AND SEA CUCUMBER Version 1.0 Genus Oncorhynchus Pacific Halibut (Hippoglossus stenolepsis) Gooseneck Barnacle (Pollicipes polymerus) Dungeness crab Sea Cucumber Spot Prawn (Cancer magister) (Apostichopus californicus) (Pandalus platyceros) Fisheries and Oceans Pêches et Océans Canada Canada This Multi-species Fishery Management Plan (FMP) is intended for general purposes only. Where there is a discrepancy between the FMP and the Fisheries Act and Regulations, the Act and Regulations are the final authority. A description of Areas and Subareas referenced in this FMP can be found in the Pacific Fishery Management Area Regulations, 2007. This FMP is not a legally binding instrument which can form the basis of a legal challenge and does not fetter the Minister’s discretionary powers set out in the Fisheries Act. 9-Apr.-21 Version 1.0 Front cover drawing (crab) by Antan Phillips, Retired Biologist, Fisheries and Oceans Canada Front cover drawing (gooseneck barnacle) by Pauline Ridings, Biologist, Fisheries and Oceans Canada Front cover drawing (sea cucumber) by Pauline Ridings, Biologist, Fisheries and Oceans Canada This page intentionally left blank 2021/22 Five Nations Multi-species Fishery Management Plan V. 1.0 Page 2 of 123 9-Apr.-21 Version 1.0 FMP Amendment Tracking Date Version Sections revised and details of revision. 2021-04-09 April 9, 2021 (1.0) Initial 2021/22 Five Nations Multi-species Fishery Management Plan V. 1.0 Page 3 of 123 9-Apr.-21 Version 1.0 CONTENTS Glossary and List of Acronyms .................................................................................................. -
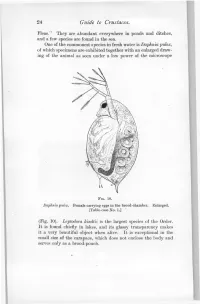
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

Investigating Genetic, Gene Expression and Proteomic Changes Over Temperature Gradients in Intertidal Nerita Species
INVESTIGATING GENETIC, GENE EXPRESSION AND PROTEOMIC CHANGES OVER TEMPERATURE GRADIENTS IN INTERTIDAL NERITA SPECIES Shorash Amin Bachelor of Biomedical Science (1A Honours) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Biomedical Sciences Faculty of Health Queensland University of Technology 2018 Investigating genetic, gene expression and proteomic changes over temperature gradients in intertidal Nerita species i Keywords De novo assembly; digital gene expression; genomics; heat shock protein; Ion torrent; transcriptome; Nerita albicilla; Nerita melanotragus; molluscs; proteome; RNAseq; thermal stress; Nerita melanotragus, Illumina. ii Investigating genetic, gene expression and proteomic changes over temperature gradients in intertidal Nerita species Abstract A key area of research in physiological genomics is understanding the gene expression and proteomic responses of specific species to abiotic change in their habitat. In order to investigate these responses, an appropriate group of organisms is required that is distributed across an environmental gradient. One such group of organisms that meet this requirement are class Gastropoda, which are distributed globally in a range of different environments. This highly speciose group are important socially, economically and ecologically. Species from this taxonomic group form a large component of intertidal zone fauna in many areas, globally. The intertidal zone is amongst the harshest of environments on Earth, with constant changes in temperature, pH, sea level and UV exposure. Furthermore, species inhabiting these areas are periodically submerged due to the tidal cycle. The intertidal zone can be further subdivided into the spray, upper, mid and lower intertidal sub zones. Abiotic stresses also vary across these habitats as does the level of submergence. -

And Taewa Māori (Solanum Tuberosum) to Aotearoa/New Zealand
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Traditional Knowledge Systems and Crops: Case Studies on the Introduction of Kūmara (Ipomoea batatas) and Taewa Māori (Solanum tuberosum) to Aotearoa/New Zealand A thesis presented in partial fulfilment of the requirement for the degree of Master of AgriScience in Horticultural Science at Massey University, Manawatū, New Zealand Rodrigo Estrada de la Cerda 2015 Kūmara and Taewa Māori, Ōhakea, New Zealand i Abstract Kūmara (Ipomoea batatas) and taewa Māori, or Māori potato (Solanum tuberosum), are arguably the most important Māori traditional crops. Over many centuries, Māori have developed a very intimate relationship to kūmara, and later with taewa, in order to ensure the survival of their people. There are extensive examples of traditional knowledge aligned to kūmara and taewa that strengthen the relationship to the people and acknowledge that relationship as central to the human and crop dispersal from different locations, eventually to Aotearoa / New Zealand. This project looked at the diverse knowledge systems that exist relative to the relationship of Māori to these two food crops; kūmara and taewa. A mixed methodology was applied and information gained from diverse sources including scientific publications, literature in Spanish and English, and Andean, Pacific and Māori traditional knowledge. The evidence on the introduction of kūmara to Aotearoa/New Zealand by Māori is indisputable. Mātauranga Māori confirms the association of kūmara as important cargo for the tribes involved, even detailing the purpose for some of the voyages. -

Human Discovery and Settlement of the Remote Easter Island (SE Pacific)
quaternary Review Human Discovery and Settlement of the Remote Easter Island (SE Pacific) Valentí Rull Laboratory of Paleoecology, Institute of Earth Sciences Jaume Almera (ICTJA-CSIC), C. Solé i Sabarís s/n, 08028 Barcelona, Spain; [email protected] Received: 19 March 2019; Accepted: 27 March 2019; Published: 2 April 2019 Abstract: The discovery and settlement of the tiny and remote Easter Island (Rapa Nui) has been a classical controversy for decades. Present-day aboriginal people and their culture are undoubtedly of Polynesian origin, but it has been debated whether Native Americans discovered the island before the Polynesian settlement. Until recently, the paradigm was that Easter Island was discovered and settled just once by Polynesians in their millennial-scale eastward migration across the Pacific. However, the evidence for cultivation and consumption of an American plant—the sweet potato (Ipomoea batatas)—on the island before the European contact (1722 CE), even prior to the Europe-America contact (1492 CE), revived controversy. This paper reviews the classical archaeological, ethnological and paleoecological literature on the subject and summarizes the information into four main hypotheses to explain the sweet potato enigma: the long-distance dispersal hypothesis, the back-and-forth hypothesis, the Heyerdahl hypothesis, and the newcomers hypothesis. These hypotheses are evaluated in light of the more recent evidence (last decade), including molecular DNA phylogeny and phylogeography of humans and associated plants and animals, physical anthropology (craniometry and dietary analysis), and new paleoecological findings. It is concluded that, with the available evidence, none of the former hypotheses may be rejected and, therefore, all possibilities remain open. -

Seacare Authority Exemption
EXEMPTION 1—SCHEDULE 1 Official IMO Year of Ship Name Length Type Number Number Completion 1 GIANT LEAP 861091 13.30 2013 Yacht 1209 856291 35.11 1996 Barge 2 DREAM 860926 11.97 2007 Catamaran 2 ITCHY FEET 862427 12.58 2019 Catamaran 2 LITTLE MISSES 862893 11.55 2000 857725 30.75 1988 Passenger vessel 2001 852712 8702783 30.45 1986 Ferry 2ABREAST 859329 10.00 1990 Catamaran Pleasure Yacht 2GETHER II 859399 13.10 2008 Catamaran Pleasure Yacht 2-KAN 853537 16.10 1989 Launch 2ND HOME 856480 10.90 1996 Launch 2XS 859949 14.25 2002 Catamaran 34 SOUTH 857212 24.33 2002 Fishing 35 TONNER 861075 9714135 32.50 2014 Barge 38 SOUTH 861432 11.55 1999 Catamaran 55 NORD 860974 14.24 1990 Pleasure craft 79 199188 9.54 1935 Yacht 82 YACHT 860131 26.00 2004 Motor Yacht 83 862656 52.50 1999 Work Boat 84 862655 52.50 2000 Work Boat A BIT OF ATTITUDE 859982 16.20 2010 Yacht A COCONUT 862582 13.10 1988 Yacht A L ROBB 859526 23.95 2010 Ferry A MORNING SONG 862292 13.09 2003 Pleasure craft A P RECOVERY 857439 51.50 1977 Crane/derrick barge A QUOLL 856542 11.00 1998 Yacht A ROOM WITH A VIEW 855032 16.02 1994 Pleasure A SOJOURN 861968 15.32 2008 Pleasure craft A VOS SANTE 858856 13.00 2003 Catamaran Pleasure Yacht A Y BALAMARA 343939 9.91 1969 Yacht A.L.S.T. JAMAEKA PEARL 854831 15.24 1972 Yacht A.M.S. 1808 862294 54.86 2018 Barge A.M.S. -

Pollination Ecology and Evolution of Epacrids
Pollination Ecology and Evolution of Epacrids by Karen A. Johnson BSc (Hons) Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy University of Tasmania February 2012 ii Declaration of originality This thesis contains no material which has been accepted for the award of any other degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Karen A. Johnson Statement of authority of access This thesis may be made available for copying. Copying of any part of this thesis is prohibited for two years from the date this statement was signed; after that time limited copying is permitted in accordance with the Copyright Act 1968. Karen A. Johnson iii iv Abstract Relationships between plants and their pollinators are thought to have played a major role in the morphological diversification of angiosperms. The epacrids (subfamily Styphelioideae) comprise more than 550 species of woody plants ranging from small prostrate shrubs to temperate rainforest emergents. Their range extends from SE Asia through Oceania to Tierra del Fuego with their highest diversity in Australia. The overall aim of the thesis is to determine the relationships between epacrid floral features and potential pollinators, and assess the evolutionary status of any pollination syndromes. The main hypotheses were that flower characteristics relate to pollinators in predictable ways; and that there is convergent evolution in the development of pollination syndromes.