Angiosperm Phylogeny Flowering Plant Systematics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cupaniopsis Anacardioides (Sapindaceae) Naturalized in Texas
Mink, J.N., J.R. Singhurst, and W.C. Holmes. 2017. Cupaniopsis anacardioides (Sapindaceae) naturalized in Texas. Phytoneuron 2017-9: 1–5. Published 1 February 2017. ISSN 2153 733X CUPANIOPSIS ANACARDIOIDES (SAPINDACEAE) NATURALIZED IN TEXAS JEFFREY N. MINK 176 Downsville Road Robinson, Texas 76706 [email protected] [email protected] JASON R. SINGHURST Wildlife Diversity Program Texas Parks and Wildlife Department 4200 Smith School Road Austin, Texas 78744 WALTER C. HOLMES Department of Biology Baylor University Waco, Texas 76798-7388 ABSTRACT Recent botanical work in Cameron Co., Texas, has resulted in the discovery of naturalized Cupaniopsis anacardioides in the understory of a Sabal mexicana-Ebenopsis ebano forest in a Nature Conservancy preserve. The population includes 30-40 plants of varying age classes from mature to seedling trees. Cupaniopsis (Sapindaceae) is a genus of about 67 species known from Australia, New Guinea, and nearby islands of Micronesia, New Caledonia, and eastern Indonesia (Morat et al. 2012; Reynolds 1985; Hyland et al. 2010). Cupaniopsis anacardioides (carrotwood, tuckeroo) is native to Australia, Indonesia, and Papua New Guinea and has been introduced to the USA –– California (Lockhart et al. 1999), Florida (Oliver 1992), Hawaii (O‘ahu, Frohlich & Lau 2010; Maui, Starr & Starr 2011; and Kauai, Starr & Starr 2015). Establishment in the USA apparently has resulted from its usage in the subtropical nursery trade during the 1950s and early 1960s. The present paper documents the occurrence of the species to Texas, based on the following specimen. TEXAS . Cameron Co.: Nature Conservancy Southmost Preserve 0.7 mi S on Southpoint Rd. from junction of FM 1419 and Southpoint Rd. -

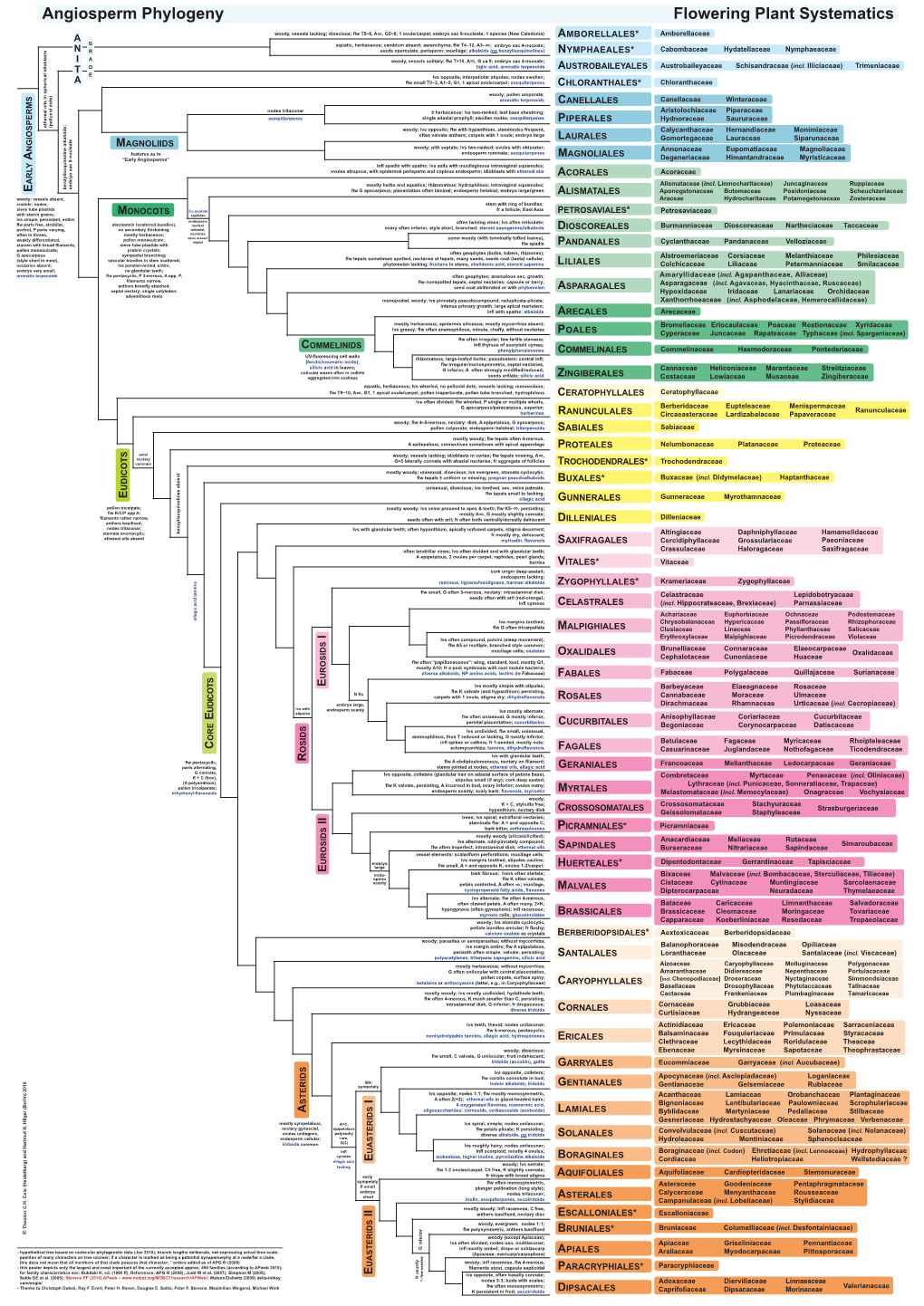
Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -

Acer Binzayedii (Sapindaceae), a New Maple Species from Mexico
Acer binzayedii (Sapindaceae), a new maple species from Mexico Yalma L. Vargas-Rodriguez, Lowell E. Urbatsch, Vesna Karaman-Castro & Blanca L. Figueroa-Rangel Brittonia ISSN 0007-196X Brittonia DOI 10.1007/s12228-017-9465-5 1 23 Your article is protected by copyright and all rights are held exclusively by The New York Botanical Garden. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Acer binzayedii (Sapindaceae), a new maple species from Mexico 1,2 1 1 YALMA L. VARGAS-RODRIGUEZ ,LOWELL E. URBATSCH ,VESNA KARAMAN-CASTRO , 3 AND BLANCA L. FIGUEROA-RANGEL 1 Department of Biological Sciences, Louisiana State University, 202 Life Sciences Building, Baton Rouge, LA 70803, USA; e-mail: [email protected] 2 National Council of Science and Technology, Av. Insurgentes Sur 1582, Col. Crédito Constructor, Ciudad de México, 03940 D.F., México 3 Department of Ecology and Natural Resources, Centro Universitario de la Costa Sur, Universidad de Guadalajara, Av. Independencia Nacional 151, 48900, Autlán de Navarro, Jalisco, México Abstract. -

Hypericaceae) Heritiana S
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 5-19-2017 Systematics, Biogeography, and Species Delimitation of the Malagasy Psorospermum (Hypericaceae) Heritiana S. Ranarivelo University of Missouri-St.Louis, [email protected] Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Botany Commons Recommended Citation Ranarivelo, Heritiana S., "Systematics, Biogeography, and Species Delimitation of the Malagasy Psorospermum (Hypericaceae)" (2017). Dissertations. 690. https://irl.umsl.edu/dissertation/690 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. Systematics, Biogeography, and Species Delimitation of the Malagasy Psorospermum (Hypericaceae) Heritiana S. Ranarivelo MS, Biology, San Francisco State University, 2010 A Dissertation Submitted to The Graduate School at the University of Missouri-St. Louis in partial fulfillment of the requirements for the degree Doctor of Philosophy in Biology with an emphasis in Ecology, Evolution, and Systematics August 2017 Advisory Committee Peter F. Stevens, Ph.D. Chairperson Peter C. Hoch, Ph.D. Elizabeth A. Kellogg, PhD Brad R. Ruhfel, PhD Copyright, Heritiana S. Ranarivelo, 2017 1 ABSTRACT Psorospermum belongs to the tribe Vismieae (Hypericaceae). Morphologically, Psorospermum is very similar to Harungana, which also belongs to Vismieae along with another genus, Vismia. Interestingly, Harungana occurs in both Madagascar and mainland Africa, as does Psorospermum; Vismia occurs in both Africa and the New World. However, the phylogeny of the tribe and the relationship between the three genera are uncertain. -

Calibrated Chronograms, Fossils, Outgroup Relationships, and Root Priors: Re-Examining the Historical Biogeography of Geraniales
bs_bs_banner Biological Journal of the Linnean Society, 2014, 113, 29–49. With 4 figures Calibrated chronograms, fossils, outgroup relationships, and root priors: re-examining the historical biogeography of Geraniales KENNETH J. SYTSMA1,*, DANIEL SPALINK1 and BRENT BERGER2 1Department of Botany, University of Wisconsin, Madison, WI 53706, USA 2Department of Biological Sciences, St. John’s University, Queens, NY 11439, USA Received 26 November 2013; revised 23 February 2014; accepted for publication 24 February 2014 We re-examined the recent study by Palazzesi et al., (2012) published in the Biological Journal of the Linnean Society (107: 67–85), that presented the historical diversification of Geraniales using BEAST analysis of the plastid spacer trnL–F and of the non-coding nuclear ribosomal internal transcribed spacers (ITS). Their study presented a set of new fossils within the order, generated a chronogram for Geraniales and other rosid orders using fossil-based priors on five nodes, demonstrated an Eocene radiation of Geraniales (and other rosid orders), and argued for more recent (Pliocene–Pleistocene) and climate-linked diversification of genera in the five recognized families relative to previous studies. As a result of very young ages for the crown of Geraniales and other rosid orders, unusual relationships of Geraniales to other rosids, and apparent nucleotide substitution saturation of the two gene regions, we conducted a broad series of BEAST analyses that incorporated additional rosid fossil priors, used more accepted rosid ordinal -

Vascular Plant Families of the United States Grouped by Diagnostic Features
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 12-6-2019 Vascular Plant Families of the United States Grouped by Diagnostic Features James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Vascular Plant Families of the United States Grouped by Diagnostic Features" (2019). Botanical Studies. 96. https://digitalcommons.humboldt.edu/botany_jps/96 This Flora of the United States and North America is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. FLOWERING PLANT FAMILIES OF THE UNITED STATES GROUPED BY DIAGNOSTIC FEATURES James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Second edition — 6 December 2019 The focus is on families of plants found in the conterminous United States, including ornamentals. The listing of a family is not meant to imply that every species has that feature. I am using a fewfamily names, such as Liliaceae, Plantaginaceae, and Scrophulariaceae, in the traditional sense, because their limits remain unsettled. Parasitic on branches Dioscoreaceae -

Flora of South Australia 5Th Edition | Edited by Jürgen Kellermann
Flora of South Australia 5th Edition | Edited by Jürgen Kellermann KEY TO FAMILIES1 J.P. Jessop2 The sequence of families used in this Flora follows closely the one adopted by the Australian Plant Census (www.anbg.gov. au/chah/apc), which in turn is based on that of the Angiosperm Phylogeny Group (APG III 2009) and Mabberley’s Plant Book (Mabberley 2008). It differs from previous editions of the Flora, which were mainly based on the classification system of Engler & Gilg (1919). A list of all families recognised in this Flora is printed in the inside cover pages with families already published highlighted in bold. The up-take of this new system by the State Herbarium of South Australia is still in progress and the S.A. Census database (www.flora.sa.gov.au/census.shtml) still uses the old classification of families. The Australian Plant Census web-site presents comparison tables of the old and new systems on family and genus level. A good overview of all families can be found in Heywood et al. (2007) and Stevens (2001–), although these authors accept a slightly different family classification. A number of names with which people using this key may be familiar but are not employed in the system used in this work have been included for convenience and are enclosed on quotation marks. 1. Plants reproducing by spores and not producing flowers (“Ferns and lycopods”) 2. Aerial shoots either dichotomously branched, with scale leaves and 3-lobed sporophores or plants with fronds consisting of a simple or divided sterile blade and a simple or branched spikelike sporophore .................................................................................. -

Crocosmia X Crocosmiiflora Montbretia Crocosmia Aurea X Crocosmia Pottsii – Naturally Occurring Hybrid
Top 40 Far Flung Flora A selection of the best plants for pollinators from the Southern Hemisphere List Curated by Thomas McBride From research data collected and collated at the National Botanic Garden of Wales NB: Butterflies and Moths are not studied at the NBGW so any data on nectar plants beneficial for them is taken from Butterfly Conservation The Southern Hemisphere Verbena bonariensis The Southern Hemisphere includes all countries below the equator. As such, those countries are the furthest from the UK and tend to have more exotic and unusual native species. Many of these species cannot be grown in the UK, but in slightly more temperate regions, some species will thrive here and be of great benefit to our native pollinators. One such example is Verbena bonariensis, native to South America, which is a big hit with our native butterfly and bumblebee species. The Southern Hemisphere contains a lower percentage of land than the northern Hemisphere so the areas included are most of South America (particularly Chile, Argentina, Ecuador and Peru), Southern Africa (particularly South Africa) and Oceania (Particularly Australia and New Zealand). A large proportion of the plants in this list are fully hardy in the UK but some are only half-hardy. Half-hardy annuals may be planted out in the spring and will flourish. Half-hardy perennials or shrubs may need to be grown in pots and moved indoors during the winter months or grown in a very sheltered location. The plants are grouped by Tropaeolum majus Continent rather than a full alphabetical -

SOAPBERRY Scientific Name: Sapindus Marginatus Willdenow
Common Name: SOAPBERRY Scientific Name: Sapindus marginatus Willdenow Other Commonly Used Names: Florida soapberry Previously Used Scientific Names: Sapindus saponaria Linnaeus Family: Sapindaceae (soapberry) Rarity Ranks: G5/S1 State Legal Status: Rare Federal Legal Status: none Federal Wetland Status: none Description: Small tree or large shrub usually less than 30 feet (10 meters) tall. Bark pale gray or brown, ridged, and warty. Leaves up to 1 foot (32 cm) long (including leaf stalk), alternate, with 6 - 13 leaflets; leaf stalk without wings between the leaflets. Leaflets 2 - 6 inches (5 - 15 cm) long and ¾ - 2¾ inches (2 - 7 cm) wide, lance-shaped with pointed tips, with no teeth along the edges; leaflets may be opposite or alternate along the stalk; leaves fall in the early spring. Flower clusters up to 7 inches (18 cm) long, with both female and male flowers. Flowers tiny, bell-shaped, with 5 pale yellow petals and 8 stamens. Fruits about ¾ inches (2 cm) long, oval but lopsided, golden-brown, leathery, wrinkled, and hard, with a large, black, poisonous seed. Similar Species: Tropical soapberry (Sapindus saponaria) is not native to Georgia, but may escape from cultivation. It has narrow wings on the leaf stalk between the leaflets; the leaflets have blunt or rounded tips, and its fruits are round. Some botanists consider Florida soapberry to be the same species as tropical soapberry. Related Rare Species: None in Georgia. Habitat: Coastal shell mounds and hardwood hammocks, often near edges of salt marsh, with live oak, red cedar, red bay, pignut hickory, and yaupon. Life History: Soapberry reproduces sexually. -

Updated Angiosperm Family Tree for Analyzing Phylogenetic Diversity and Community Structure
Acta Botanica Brasilica - 31(2): 191-198. April-June 2017. doi: 10.1590/0102-33062016abb0306 Updated angiosperm family tree for analyzing phylogenetic diversity and community structure Markus Gastauer1,2* and João Augusto Alves Meira-Neto2 Received: August 19, 2016 Accepted: March 3, 2017 . ABSTRACT Th e computation of phylogenetic diversity and phylogenetic community structure demands an accurately calibrated, high-resolution phylogeny, which refl ects current knowledge regarding diversifi cation within the group of interest. Herein we present the angiosperm phylogeny R20160415.new, which is based on the topology proposed by the Angiosperm Phylogeny Group IV, a recently released compilation of angiosperm diversifi cation. R20160415.new is calibratable by diff erent sets of recently published estimates of mean node ages. Its application for the computation of phylogenetic diversity and/or phylogenetic community structure is straightforward and ensures the inclusion of up-to-date information in user specifi c applications, as long as users are familiar with the pitfalls of such hand- made supertrees. Keywords: angiosperm diversifi cation, APG IV, community tree calibration, megatrees, phylogenetic topology phylogeny comprising the entire taxonomic group under Introduction study (Gastauer & Meira-Neto 2013). Th e constant increase in knowledge about the phylogenetic The phylogenetic structure of a biological community relationships among taxa (e.g., Cox et al. 2014) requires regular determines whether species that coexist within a given revision of applied phylogenies in order to incorporate novel data community are more closely related than expected by chance, and is essential information for investigating and avoid out-dated information in analyses of phylogenetic community assembly rules (Kembel & Hubbell 2006; diversity and community structure. -

RHS Seed Exchange 2020
RHS Seed Exchange rhs.org.uk/seedlist Introduction to RHS Seed Exchange 2121 The Royal Horticultural Society is the UK’s Dispatch of Orders leading gardening charity, which aims to enrich We will start to send out orders from January everyone’s life through plants, and make the UK a 2020 and dispatch is usually completed by the greener and more beautiful place. This vision end of April. If you have not received your seed underpins all that we do, from inspirational by 1st May please contact us by email: gardens and shows, through our scientific [email protected] research, to our education and community programmes. We’re committed to inspiring Convention on Biological Diversity everyone to grow. 3Nagoya Protocol4 In accordance with the Convention on Biological Most of the seed offered is collected in RHS Diversity (CBD), the Royal Horticultural Society Gardens. Other seed is donated and is offered supplies seed from its garden collections on the under the name provided by the donor. In many conditions that: cases only limited quantities of seed are available. ⅷ The plant material is used for the common However, we feel that even small quantities good in areas of research, education, should be distributed if at all possible. conservation and the development of horticultural institutions or gardens. Our seed is collected from open-pollinated If the recipient seeks to commercialise the plants, therefore may not come true. ⅷ genetic material, its products or resources derived from it, then written permission must Please note we are only able to send seed to be sought from the Royal Horticultural addresses in the UK and EU6 including Society.