Title of the Opinion
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vitis Vinifera L.)
UNIVERSIDAD POLITÉCNICA DE CARTAGENA DEPARTAMENTO DE CIENCIA Y TECNOLOGÍA AGRARIA GENETIC TRANSFORMATION AND ELICITATION TO OBTAIN MEDICINAL COMPOUNDS IN GRAPEVINE ( Vitis vinifera L.) AND IN Bituminaria bituminosa (L.) STIRT. María Pazos Navarro 2012 UNIVERSIDAD POLITÉCNICA DE CARTAGENA DEPARTAMENTO DE CIENCIA Y TECNOLOGÍA AGRARIA GENETIC TRANSFORMATION AND ELICITATION TO OBTAIN MEDICINAL COMPOUNDS IN GRAPEVINE ( Vitis vinifera L.) AND IN Bituminaria bituminosa (L.) STIRT. María Pazos Navarro Directora Mercedes Dabauza Micó 2012 Acknowledgements ACKNOWLEDGEMENTS Me gustaría dar las gracias a todas aquellas personas que han tenido algo que ver en la realización de esta tesis, ya sea de manera directa o indirecta. Espero no olvidar mencionar a nadie… Primero de todo, quiero agradecer a mi directora de tesis, la Dra. Mercedes Dabauza, su esfuerzo y paciencia durante la realización de esta tesis. Al final de todo seguimos llevándonos muy bien, y puedo decir que además de una gran directora de tesis, es una muy buena amiga. Muchas gracias por todo. Elena, Domingo y Antonio muchas gracias por esos viajes a Cartagena a las clases del Master. Entre todos hacíamos menos aburridos esos viajes. No puedo olvidarme del Equipo de Fruticultura del IMIDA; que puedo decir de ell@s: Pepe Cos y Antonio Carrillo, lo que me he reido y lo bien que me lo he pasado con vosotros emasculando flores; muchísimas gracias por esos buenos recuerdos, hacéis un buen tándem, seguid así. Marga, amiga mía, después de tantos años creo que nos lo hemos dicho casi todo; así que solo te digo que ¡dentro de poco te tocará a ti! Ten paciencia. -

Appendix Color Plates of Solanales Species
Appendix Color Plates of Solanales Species The first half of the color plates (Plates 1–8) shows a selection of phytochemically prominent solanaceous species, the second half (Plates 9–16) a selection of convol- vulaceous counterparts. The scientific name of the species in bold (for authorities see text and tables) may be followed (in brackets) by a frequently used though invalid synonym and/or a common name if existent. The next information refers to the habitus, origin/natural distribution, and – if applicable – cultivation. If more than one photograph is shown for a certain species there will be explanations for each of them. Finally, section numbers of the phytochemical Chapters 3–8 are given, where the respective species are discussed. The individually combined occurrence of sec- ondary metabolites from different structural classes characterizes every species. However, it has to be remembered that a small number of citations does not neces- sarily indicate a poorer secondary metabolism in a respective species compared with others; this may just be due to less studies being carried out. Solanaceae Plate 1a Anthocercis littorea (yellow tailflower): erect or rarely sprawling shrub (to 3 m); W- and SW-Australia; Sects. 3.1 / 3.4 Plate 1b, c Atropa belladonna (deadly nightshade): erect herbaceous perennial plant (to 1.5 m); Europe to central Asia (naturalized: N-USA; cultivated as a medicinal plant); b fruiting twig; c flowers, unripe (green) and ripe (black) berries; Sects. 3.1 / 3.3.2 / 3.4 / 3.5 / 6.5.2 / 7.5.1 / 7.7.2 / 7.7.4.3 Plate 1d Brugmansia versicolor (angel’s trumpet): shrub or small tree (to 5 m); tropical parts of Ecuador west of the Andes (cultivated as an ornamental in tropical and subtropical regions); Sect. -

Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Spring 5-2016 Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi Hanna M. Miller University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Biodiversity Commons, and the Botany Commons Recommended Citation Miller, Hanna M., "Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi" (2016). Honors Theses. 389. https://aquila.usm.edu/honors_theses/389 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Vascular Flora of the Possum Walk Trail at the Infinity Science Center, Hancock County, Mississippi by Hanna Miller A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirement for the Degree of Bachelor of Science in the Department of Biological Sciences May 2016 ii Approved by _________________________________ Mac H. Alford, Ph.D., Thesis Adviser Professor of Biological Sciences _________________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences _________________________________ Ellen Weinauer, Ph.D., Dean Honors College iii Abstract The North American Coastal Plain contains some of the highest plant diversity in the temperate world. However, most of the region has remained unstudied, resulting in a lack of knowledge about the unique plant communities present there. -

A New Application for Phylogenetic Marker Development Using Angiosperm Transcriptomes Author(S): Srikar Chamala, Nicolás García, Grant T
MarkerMiner 1.0: A New Application for Phylogenetic Marker Development Using Angiosperm Transcriptomes Author(s): Srikar Chamala, Nicolás García, Grant T. Godden, Vivek Krishnakumar, Ingrid E. Jordon- Thaden, Riet De Smet, W. Brad Barbazuk, Douglas E. Soltis, and Pamela S. Soltis Source: Applications in Plant Sciences, 3(4) Published By: Botanical Society of America DOI: http://dx.doi.org/10.3732/apps.1400115 URL: http://www.bioone.org/doi/full/10.3732/apps.1400115 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. ApApplicatitionsons Applications in Plant Sciences 2015 3 ( 4 ): 1400115 inin PlPlant ScienSciencesces S OFTWARE NOTE M ARKERMINER 1.0: A NEW APPLICATION FOR PHYLOGENETIC 1 MARKER DEVELOPMENT USING ANGIOSPERM TRANSCRIPTOMES S RIKAR C HAMALA 2,12 , N ICOLÁS G ARCÍA 2,3,4 * , GRANT T . G ODDEN 2,3,5 * , V IVEK K RISHNAKUMAR 6 , I NGRID E. -

Vegetation Succession Along New Roads at Soqotra Island (Yemen): Effects of Invasive Plant Species and Utilization of Selected N
10.2478/jlecol-2014-0003 Journal of Landscape Ecology (2013), Vol: 6 / No. 3. VEGETATION SUCCESSION ALONG NEW ROADS AT SOQOTRA ISLAND (YEMEN): EFFECTS OF INVASIVE PLANT SPECIES AND UTILIZATION OF SELECTED NATIVE PLANT RESISTENCE AGAINST DISTURBANCE PETR MADĚRA1, PAVEL KOVÁŘ2, JAROSLAV VOJTA2, DANIEL VOLAŘÍK1, LUBOŠ ÚRADNÍČEK1, ALENA SALAŠOVÁ3, JAROSLAV KOBLÍŽEK1 & PETR JELÍNEK1 1Mendel University in Brno, Faculty of Forestry and Wood Technology, Department of the Forest Botany, Dendrology and Geobiocoenology, Zemědělská 1/1665, 613 00 Brno 2Charles University in Prague, Faculty of Science, Department of Botany, Benátská 2, 128 01 Prague 3Mendel University in Brno, Faculty of Horticulture, Department of Landscape Planning, Valtická 337, 691 44 Lednice Received: 13th November 2013, Accepted: 17th December 2013 ABSTRACT The paved (tarmac) roads had been constructed on Soqotra island over the last 15 years. The vegetation along the roads was disturbed and the erosion started immediately after the disturbance caused by the road construction. Our assumption is that biotechnical measurements should prevent the problems caused by erosion and improve stabilization of road edges. The knowledge of plant species which are able to grow in unfavourable conditions along the roads is important for correct selection of plants used for outplanting. The vegetation succession was observed using phytosociological relevés as a tool of recording and mapping assambblages of plants species along the roads as new linear structures in the landscape. Data from phytosociological relevés were analysed and the succession was characterised in different altitudes. The results can help us to select group of plants (especially shrubs and trees), which are suitable to be used as stabilizing green mantle in various site conditions and for different purposes (anti-erosional, ornamental, protection against noise or dust, etc.). -

In Wadi Allaqi, Egypt
ENVIRONMENTAL VALUATION AND MANAGEMENT OF PLANTS IN WADI ALLAQI, EGYPT FINAL REPORT IDRC OQ w W1.44 Trent University AUGUST 1998 ENVIRONMENTAL VALUATION AND-MANAGEMENT OF PLANTS IN WADI ALLAQI, EGYPT Final report Editors: Belal, A.E. , B. Leith, J. Solway and 1. Springuel Submitted To INTERNATIONAL DEVELOPMENT RESEARCH CENTRE (IDRC) CANADA File: 95-100"1/02 127-01 UNIT OF ENVIRONMENTAL STUDIES AND DEVELOPMENT, SOUTH VALLEY UNIVERSITY, ASWAN, EGYPT A-RC hf v 5 91, 5 7 By Acknowledgements The Project team of both South Valley and Trent Universities wish to thank the International Development Research Center (IDRC) Ottawa, Canada, for supporting the project with funding and for visiting the site. We also thank the staff of the IDRC Cairo Office for their assistance. This report is based upon the knowledge, hard work, and support of many people and institutions. We thank the British Council for the support they have provided in training many members of the team and UNESCO for providing support for the Allaqi project and Biosphere Reserve. We appreciate the good working relationship that we have developed with the Egyptian Environment Affairs Agency. Dr. M. Kassas of Cairo University has provided valuable intellectual direction for the project. We thank C. Fararldi who has assisted the project in numerous ways and Gordon Dickinson for writing notes on establishing the visitor center in Wadi Allaqi We wish to thank the research offices of Trent University and South Valley University. We are deeply grateful to the residents of Wadi Allaqi for their help and continued support and patience towards our project. -

Reproductive Phasirna Loci and DICER-LIKE5, but Not Microrna
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.25.061721; this version posted April 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Reproductive phasiRNA loci and DICER‐LIKE5, but not microRNA loci, diversified in monocotyledonous plants Parth Patel2§, Sandra M. Mathioni1§, Reza Hammond2, Alex E. Harkess1, Atul Kakrana2, Siwaret Arikit3, Ayush Dusia2, and Blake C. Meyers1,2,4* 1Donald Danforth Plant Science Center, Saint Louis, Missouri 63132 2Center for Bioinformatics and Computational Biology, Delaware Biotechnology Institute, University of Delaware, Newark, Delaware 19716 3Department of Agronomy, Kamphaeng Saen and Rice Science Center, Kasetsart University, Nakhon Pathom 73140, Thailand 4Division of Plant Sciences, University of Missouri, Columbia, Missouri 65211 §These authors contributed equally to this work. *Corresponding author: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.04.25.061721; this version posted April 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Abstract (200 words) 2 In monocots other than maize and rice, the repertoire and diversity of microRNAs (miRNAs) and 3 the populations of phased, secondary, small interfering RNAs (phasiRNAs) are poorly 4 characterized. To remedy this, we sequenced small RNAs from vegetative and dissected 5 inflorescence tissue in 28 phylogenetically diverse monocots and from several early‐diverging 6 angiosperm lineages, as well as publicly available data from 10 additional monocot species. -

Low Risk Aquarium and Pond Plants
Plant Identification Guide Low-risk aquarium and pond plants Planting these in your pond or aquarium is environmentally-friendly. Glossostigma elatinoides, image © Sonia Frimmel. One of the biggest threats to New Zealand’s waterbodies is the establishment and proliferation of weeds. The majority of New Zealand’s current aquatic weeds started out as aquarium and pond plants. To reduce the occurrence of new weeds becoming established in waterbodies this guide has been prepared to encourage the use of aquarium and pond plants that pose minimal risk to waterbodies. Guide prepared by Dr John Clayton, Paula Reeves, Paul Champion and Tracey Edwards, National Centre of Aquatic Biodiversity and Biosecurity, NIWA with funding from the Department of Conservation. The guides will be updated on a regular basis and will be available on the NIWA website: www.niwa.co.nz/ncabb/tools. Key to plant life-forms Sprawling marginal plants. Grow across the ground and out over water. Pond plants Short turf-like plants. Grow in shallow water on the edges of ponds and foreground of aquariums. Includes very small plants (up to 2-3 cm in height). Most species can grow both submerged (usually more erect) and emergent. Pond and aquarium plants Tall emergent plants. Can grow in water depths up to 2 m deep depending on the species. Usually tall reed-like plants but sometimes with broad leaves. Ideal for deeper ponds. Pond plants Free floating plants. These plants grow on the water surface and are not anchored to banks or bottom substrates. Pond and aquarium plants Floating-leaved plants. Water lily-type plants. -

Observational Evidence on the Effects of Mega-Fires on the Frequency Of
Science of the Total Environment 592 (2017) 262–276 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Observational evidence on the effects of mega-fires on the frequency of hydrogeomorphic hazards. The case of the Peloponnese fires of 2007 in Greece Diakakis M. a,⁎, Nikolopoulos E.I. b,MavroulisS.a,VassilakisE.a,KorakakiE.c a Faculty of Geology and Geoenvironment, National & Kapodistrian University of Athens, Panepistimioupoli, Zografou GR15784, Greece b Department of Civil and Environmental Engineering, University of Connecticut, Storrs, CT, USA c WWF Greece, 21 Lembessi St., 117 43 Athens, Greece HIGHLIGHTS GRAPHICAL ABSTRACT • The mega fire of 2007 in Greece and its effects of hydrogeomorphic events are studied. • The frequency of such events over the period 1989–2016 is examined. • Results show an increase in floods by 3.3 times and mass movement events by 5.6. • Increase in frequency of such events is steeper in affected areas than unaf- fected. • Increases are found even in months that record a decrease in extreme rainfall. article info abstract Article history: Even though rare, mega-fires raging during very dry and windy conditions, record catastrophic impacts on infra- Received 6 January 2017 structure, the environment and human life, as well as extremely high suppression and rehabilitation costs. Apart Received in revised form 7 March 2017 from the direct consequences, mega-fires induce long-term effects in the geomorphological and hydrological Accepted 8 March 2017 processes, influencing environmental factors that in turn can affect the occurrence of other natural hazards, Available online xxxx such as floods and mass movement phenomena. -

Recerca I Territori V12 B (002)(1).Pdf
Butterfly and moths in l’Empordà and their response to global change Recerca i territori Volume 12 NUMBER 12 / SEPTEMBER 2020 Edition Graphic design Càtedra d’Ecosistemes Litorals Mediterranis Mostra Comunicació Parc Natural del Montgrí, les Illes Medes i el Baix Ter Museu de la Mediterrània Printing Gràfiques Agustí Coordinadors of the volume Constantí Stefanescu, Tristan Lafranchis ISSN: 2013-5939 Dipòsit legal: GI 896-2020 “Recerca i Territori” Collection Coordinator Printed on recycled paper Cyclus print Xavier Quintana With the support of: Summary Foreword ......................................................................................................................................................................................................... 7 Xavier Quintana Butterflies of the Montgrí-Baix Ter region ................................................................................................................. 11 Tristan Lafranchis Moths of the Montgrí-Baix Ter region ............................................................................................................................31 Tristan Lafranchis The dispersion of Lepidoptera in the Montgrí-Baix Ter region ...........................................................51 Tristan Lafranchis Three decades of butterfly monitoring at El Cortalet ...................................................................................69 (Aiguamolls de l’Empordà Natural Park) Constantí Stefanescu Effects of abandonment and restoration in Mediterranean meadows .......................................87 -

Ganas, A., Serpelloni, E., Drakatos, G., Kolligri, M., Adamis, I., Tsimi, Ch
This article was downloaded by: [HEAL-Link Consortium] On: 20 October 2009 Access details: Access Details: [subscription number 772810551] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Journal of Earthquake Engineering Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t741771161 The Mw 6.4 SW-Achaia (Western Greece) Earthquake of 8 June 2008: Seismological, Field, GPS Observations, and Stress Modeling A. Ganas a; E. Serpelloni b; G. Drakatos a; M. Kolligri a; I. Adamis a; Ch. Tsimi a; E. Batsi a a Institute of Geodynamics, National Observatory of Athens, Athens, Greece b Istituto Nazionale di Geofisica e Vulcanologia, Centro Nazionale Terremoti, Bologna, Italy Online Publication Date: 01 December 2009 To cite this Article Ganas, A., Serpelloni, E., Drakatos, G., Kolligri, M., Adamis, I., Tsimi, Ch. and Batsi, E.(2009)'The Mw 6.4 SW- Achaia (Western Greece) Earthquake of 8 June 2008: Seismological, Field, GPS Observations, and Stress Modeling',Journal of Earthquake Engineering,13:8,1101 — 1124 To link to this Article: DOI: 10.1080/13632460902933899 URL: http://dx.doi.org/10.1080/13632460902933899 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -
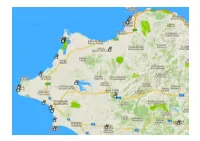
22-Nord-West.Pdf
Ort Wo Koordinaten Beschrieb Patras-1 (Valtos) (Richt. Athen). 38.28111, 21.75111 Beachside parking to the north of Patras. Midway between Patras and the Rio Bridge. Approach from SW (Patras) only, as there are "No Entry" signs from East. Beach bars with water taps. Alte Küststrasse fahren Acropolis-Travel Patras. 38.25918, 21.73874 Acropolis Travel Patras – Reisebüro ist neu hier 38.259186 21.738749 Iroon Polytechneioy & 1 Thessalonilkis Srt Patras 264 41 Griechenland Tel. +30 697 329 1605 Mail: Acropolis Travel <[email protected]> Patras-2 (Marina). 38.26308, 21.7389 Overnight parking on marina road which runs parallel to main road on sea side. Parking in various spots mostly at N end. Water taps all along face of quay wall towards the boats. Noisy. Lit. Bars nearby and excellent restaurant called Navpigeio (?????????) on opposite side of main road. To the left of the Hilti showroom. Patras-3 Gas füllen Nähe Patras. 38.10416, 21.63555 Ob das heute noch möglich ist ?? Kalogria-1 (Camper-Stop) (10€) 38.15968, 21.37158 V+E, Strom, WiFi usw. 10€. Nur 1 Std. nach Patras Kalogria-2 ( Beach Mid). 38.15223, 21.36885 Just to the south of the main wildcamping spot. Quieter and closer to the beach. Kalogria-3 (Beach North) 38.15659, 21.36793 Huge Parking area next to lovely sandy beach. Good taverna just along the road. WoMo-Kollege "Toni" warnt: Ja - die beiden Plätze Kalogria Nord und Mid sind aus meiner Sicht nicht mehr zu gebrauchen. Leider. Beide waren sehr beliebt. Es gab mehrere Vorkommnisse mit Belästigung und Diebstahl durch Zigeuner.