Drug Index and Glossary
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

PRODUCT MONOGRAPH ORCIPRENALINE Orciprenaline Sulphate Syrup House Standard 2 Mg/Ml Β2-Adrenergic Stimulant Bronchodilator AA P
PRODUCT MONOGRAPH ORCIPRENALINE Orciprenaline Sulphate Syrup House Standard 2 mg/mL 2-Adrenergic Stimulant Bronchodilator AA PHARMA INC. DATE OF PREPARATION: 1165 Creditstone Road, Unit #1 April 10, 2014 Vaughan, Ontario L4K 4N7 Control Number: 172362 1 PRODUCT MONOGRAPH ORCIPRENALINE Orciprenaline Sulfate Syrup House Standard 2 mg/mL THERAPEUTIC CLASSIFICATION 2–Adrenergic Stimulant Bronchodilator ACTIONS AND CLINICAL PHARMACOLOGY Orciprenaline sulphate is a bronchodilating agent. The bronchospasm associated with various pulmonary diseases - chronic bronchitis, pulmonary emphysema, bronchial asthma, silicosis, tuberculosis, sarcoidosis and carcinoma of the lung, has been successfully reversed by therapy with orciprenaline sulphate. Orciprenaline sulphate has the following major characteristics: 1) Pharmacologically, the action of orciprenaline sulphate is one of beta stimulation. Receptor sites in the bronchi and bronchioles are more sensitive to the drug than those in the heart and blood vessels, so that the ratio of bronchodilating to cardiovascular effects is favourable. Consequently, it is usually possible clinically to produce good bronchodilation at dosage levels which are unlikely to cause cardiovascular side effects. 2 2) The efficacy of the bronchodilator after both oral and inhalation administration has been demonstrated by pulmonary function studies (spirometry, and by measurement of airways resistance by body plethysmography). 3) Rapid onset of action follows administration of orciprenaline sulphate inhalants, and the effect is usually noted immediately. Following oral administration, the effect is usually noted within 30 minutes. 4) The peak effect of bronchodilator activity following orciprenaline sulphate generally occurs within 60 to 90 minutes, and this activity lasts for 3 to 6 hours. 5) Orciprenaline sulphate taken orally potentiates the action of a bronchodilator inhalant administered 90 minutes later, whereas no additive effect occurs when the drugs are given in reverse order. -

Infant Antibiotic Exposure Search EMBASE 1. Exp Antibiotic Agent/ 2
Infant Antibiotic Exposure Search EMBASE 1. exp antibiotic agent/ 2. (Acedapsone or Alamethicin or Amdinocillin or Amdinocillin Pivoxil or Amikacin or Aminosalicylic Acid or Amoxicillin or Amoxicillin-Potassium Clavulanate Combination or Amphotericin B or Ampicillin or Anisomycin or Antimycin A or Arsphenamine or Aurodox or Azithromycin or Azlocillin or Aztreonam or Bacitracin or Bacteriocins or Bambermycins or beta-Lactams or Bongkrekic Acid or Brefeldin A or Butirosin Sulfate or Calcimycin or Candicidin or Capreomycin or Carbenicillin or Carfecillin or Cefaclor or Cefadroxil or Cefamandole or Cefatrizine or Cefazolin or Cefixime or Cefmenoxime or Cefmetazole or Cefonicid or Cefoperazone or Cefotaxime or Cefotetan or Cefotiam or Cefoxitin or Cefsulodin or Ceftazidime or Ceftizoxime or Ceftriaxone or Cefuroxime or Cephacetrile or Cephalexin or Cephaloglycin or Cephaloridine or Cephalosporins or Cephalothin or Cephamycins or Cephapirin or Cephradine or Chloramphenicol or Chlortetracycline or Ciprofloxacin or Citrinin or Clarithromycin or Clavulanic Acid or Clavulanic Acids or clindamycin or Clofazimine or Cloxacillin or Colistin or Cyclacillin or Cycloserine or Dactinomycin or Dapsone or Daptomycin or Demeclocycline or Diarylquinolines or Dibekacin or Dicloxacillin or Dihydrostreptomycin Sulfate or Diketopiperazines or Distamycins or Doxycycline or Echinomycin or Edeine or Enoxacin or Enviomycin or Erythromycin or Erythromycin Estolate or Erythromycin Ethylsuccinate or Ethambutol or Ethionamide or Filipin or Floxacillin or Fluoroquinolones -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -
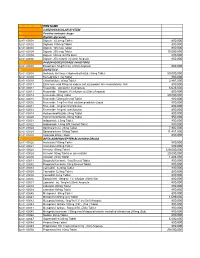
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Clinical Trial of Orciprenaline in Bradyarrhythmias
100 SINGAPORE MEDICAL JOURNAL Vol. 16, No. 2. June, 1975. CLINICAL TRIAL OF ORCIPRENALINE IN BRADYARRHYTHMIAS By Chin Hock, Lirn, Charles C. S. Toh and Oon Teik, Khoo SYNOPSIS The intravenous and oral forms of orciprenaline was used in the treatment of patients with severe sinus bradycardia, high grade atrio -ventricular block or the sick sinus syndrome. Intra- venous orciprenaline can be used to tide patients over a period of severe bradyarrhythmia, or prior to cardiac pacing. One patient developed ventricular tachycardia following an intravenous bolus of orciprenaline. Oral orciprenaline was used in 23 patients with severe bradyarrhythmia. Two patients responded poorly to it. Saventrine was given sequentially in nine patients. The merits and side effects of both drugs are discussed. INTRODUCTION efficiency of orciprenaline and to compare it with Saventrine in the treatment of complete In 1952, Nathanson and Miller introduced heart block and other forms of bradyarrhyth- isoprenaline for the treatment of heart block and mias. it still has an important role in the treatment of bradyarrhythmias. Nis Innissen and Thompson PHARMACOLOGY (1965) felt that the use of a sustained release form of isoprenaline could reduce the need for Orciprenaline (Alupent*) is a derivative of pacemakers. Intravenous isoprenaline and orci- isoprenaline but it has predominant beta -two prenaline may be used to treat patients with stimulatine effects and is commonly used as a Stokes Adams (S.A.) attacks resulting from very bronchodilator (Bogdan, 1969). However, it has slow ventricular rates in acute myocardial chronotropic and inotropic effects. It stimulates infarction, either alone or prior to cardiac the sino -atrial and atrioventricular nodes and pacing. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Pharmaceuticals Appendix
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ADAPALENE 106685-40-9 ABANOQUIL 90402-40-7 ADAPROLOL 101479-70-3 ABECARNIL 111841-85-1 ADEMETIONINE 17176-17-9 ABLUKAST 96566-25-5 ADENOSINE PHOSPHATE 61-19-8 ABUNIDAZOLE 91017-58-2 ADIBENDAN 100510-33-6 ACADESINE 2627-69-2 ADICILLIN 525-94-0 ACAMPROSATE 77337-76-9 ADIMOLOL 78459-19-5 ACAPRAZINE 55485-20-6 ADINAZOLAM 37115-32-5 ACARBOSE 56180-94-0 ADIPHENINE 64-95-9 ACEBROCHOL 514-50-1 ADIPIODONE 606-17-7 ACEBURIC ACID 26976-72-7 ADITEREN 56066-19-4 ACEBUTOLOL 37517-30-9 ADITOPRIME 56066-63-8 ACECAINIDE 32795-44-1 ADOSOPINE 88124-26-9 ACECARBROMAL 77-66-7 ADOZELESIN 110314-48-2 ACECLIDINE 827-61-2 ADRAFINIL 63547-13-7 ACECLOFENAC 89796-99-6 ADRENALONE 99-45-6 ACEDAPSONE 77-46-3 AFALANINE 2901-75-9 ACEDIASULFONE SODIUM 127-60-6 AFLOQUALONE 56287-74-2 ACEDOBEN 556-08-1 AFUROLOL 65776-67-2 ACEFLURANOL 80595-73-9 AGANODINE 86696-87-9 ACEFURTIAMINE 10072-48-7 AKLOMIDE 3011-89-0 ACEFYLLINE CLOFIBROL 70788-27-1 -

Orciprenaline Sulphate (Alupent): Planned Withdrawal from the UK Market Following a Risk-Benefit Analysis
MHRA PUBLIC ASSESSMENT REPORT Orciprenaline sulphate (Alupent): planned withdrawal from the UK market following a risk-benefit analysis November 2009 Executive summary 2 1. Introduction 3 2. Summary of data 3 2.1 Clinical pharmacology 3 2.2 Efficacy 3 2.3 Safety 4 3. Conclusions 8 4. References 9 5. Glossary 10 1 EXECUTIVE SUMMARY (Please note that this summary is intended to be accessible to all members of the public, including health professionals) Background The Medicines and Healthcare products Regulatory Agency (MHRA) is the government agency responsible for regulating the effectiveness and safety of medicines and medical devices in the UK. We continually review the safety of all medicines in the UK, and inform healthcare professionals and the public of the latest safety updates. In our Public Assessment Reports, we discuss the evidence for a safety issue with a particular drug or drug class, and changes made to the product information for the drug on the basis of this evidence, which will help safeguard public health. This MHRA Public Assessment Report discusses a review of the risks and benefits of a medicine called orciprenaline sulphate. Orciprenaline sulphate is available for oral administration as a syrup used to treat reversible airways obstructiona, which is a symptom of asthmab and chronic obstructive pulmonary diseasec. It acts on specific areas in the body called β- receptors, which relaxes the muscles used for breathing and opens the airways in the lungs. Orciprenaline sulphate was licensed in 1972 and is marketed in the UK under the brand name Alupent Syrup. As with any medicine, the use of orciprenaline sulphate may lead to adverse reactions (side-effects) in some individuals, which are described in the product information, including the patient information leaflet (see the Electronic Medicines Compendium (product information) website). -

E3 Appendix 1 (Part 1 of 2): Search Strategy Used in MEDLINE
This single copy is for your personal, non-commercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, contact CJHP at [email protected] Appendix 1 (part 1 of 2): Search strategy used in MEDLINE # Searches 1 exp *anti-bacterial agents/ or (antimicrobial* or antibacterial* or antibiotic* or antiinfective* or anti-microbial* or anti-bacterial* or anti-biotic* or anti- infective* or “ß-lactam*” or b-Lactam* or beta-Lactam* or ampicillin* or carbapenem* or cephalosporin* or clindamycin or erythromycin or fluconazole* or methicillin or multidrug or multi-drug or penicillin* or tetracycline* or vancomycin).kf,kw,ti. or (antimicrobial or antibacterial or antiinfective or anti-microbial or anti-bacterial or anti-infective or “ß-lactam*” or b-Lactam* or beta-Lactam* or ampicillin* or carbapenem* or cephalosporin* or c lindamycin or erythromycin or fluconazole* or methicillin or multidrug or multi-drug or penicillin* or tetracycline* or vancomycin).ab. /freq=2 2 alamethicin/ or amdinocillin/ or amdinocillin pivoxil/ or amikacin/ or amoxicillin/ or amphotericin b/ or ampicillin/ or anisomycin/ or antimycin a/ or aurodox/ or azithromycin/ or azlocillin/ or aztreonam/ or bacitracin/ or bacteriocins/ or bambermycins/ or bongkrekic acid/ or brefeldin a/ or butirosin sulfate/ or calcimycin/ or candicidin/ or capreomycin/ or carbenicillin/ or carfecillin/ or cefaclor/ or cefadroxil/ or cefamandole/ or cefatrizine/ or cefazolin/ or cefixime/ or cefmenoxime/ or cefmetazole/ or cefonicid/ or cefoperazone/ -

Product Innovation and Differentiation, Intra-Industry Trade and Growth
l$.to- oz- Product Innovation and Differentiation, Intra-IndustrY Trade and Growth A thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy by Geoffrey Peak The School of Economics The UniversitY of Adelaide September 2001 Table of Contents l.Introduction 1 2. Innovation and Growth: Literature Review 5 2.1 Introduction 5 2.2 Trade in Differentiated Products 5 2.3 Transport Costs... 7 2.4Trude in Differentiated Products and an "Outside Good" ..' 7 2.5 Innovation and Growth. 9 2.5. I Innovation Without Considering Trade ...'. 0 2.5.2 Innovation and Trade............. 2.5.3 Returns to Factors 2.5.3.I Trade Between Dissimilar Countries.' 2.5.3.2 Trade Between Similar Countries.. 2.5.3.3 Conclusions on Factor Proportions ....... 2.6 Conclusions 3. Innovation, Differentiation and Growth: The Underlying Logic and Arguments ........ 19 3.1 Introduction .... T9 19 3 .2 Differentiated Goods 3.3 Innovative Goods. 2t 3.4 Innovative and Differentiated Goods 27 3.4.1 Innovative and Differentiated - The Distinction 21 3.4.2 Innovation Leads to Differentiation 32 3.4.3 Differentiation Requires Innovation 32 3.4.4Penicillin..... ..JJ 3 .4.4.1 The Development of Penicillin'.........'...'.. 34 3.4.4.2 Penicillin Substitutes and Product Variety 39 3.4.4.3 Conditions for Trade Driven by Product Differentiation ....... 46 3.4.4.4 Conclusions ......... 48 3.5 Innovation, Differentiation, and Import Demand 50 3.5. 1 Consumption Goods .. 50 52 3 .5 .2 Intermediate Goods 3.6 Model of Trade in Differentiated Goods .53 3.7 Trade and Growth 63 3.7.