WO 2008/150493 Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PRODUCT MONOGRAPH ORCIPRENALINE Orciprenaline Sulphate Syrup House Standard 2 Mg/Ml Β2-Adrenergic Stimulant Bronchodilator AA P
PRODUCT MONOGRAPH ORCIPRENALINE Orciprenaline Sulphate Syrup House Standard 2 mg/mL 2-Adrenergic Stimulant Bronchodilator AA PHARMA INC. DATE OF PREPARATION: 1165 Creditstone Road, Unit #1 April 10, 2014 Vaughan, Ontario L4K 4N7 Control Number: 172362 1 PRODUCT MONOGRAPH ORCIPRENALINE Orciprenaline Sulfate Syrup House Standard 2 mg/mL THERAPEUTIC CLASSIFICATION 2–Adrenergic Stimulant Bronchodilator ACTIONS AND CLINICAL PHARMACOLOGY Orciprenaline sulphate is a bronchodilating agent. The bronchospasm associated with various pulmonary diseases - chronic bronchitis, pulmonary emphysema, bronchial asthma, silicosis, tuberculosis, sarcoidosis and carcinoma of the lung, has been successfully reversed by therapy with orciprenaline sulphate. Orciprenaline sulphate has the following major characteristics: 1) Pharmacologically, the action of orciprenaline sulphate is one of beta stimulation. Receptor sites in the bronchi and bronchioles are more sensitive to the drug than those in the heart and blood vessels, so that the ratio of bronchodilating to cardiovascular effects is favourable. Consequently, it is usually possible clinically to produce good bronchodilation at dosage levels which are unlikely to cause cardiovascular side effects. 2 2) The efficacy of the bronchodilator after both oral and inhalation administration has been demonstrated by pulmonary function studies (spirometry, and by measurement of airways resistance by body plethysmography). 3) Rapid onset of action follows administration of orciprenaline sulphate inhalants, and the effect is usually noted immediately. Following oral administration, the effect is usually noted within 30 minutes. 4) The peak effect of bronchodilator activity following orciprenaline sulphate generally occurs within 60 to 90 minutes, and this activity lasts for 3 to 6 hours. 5) Orciprenaline sulphate taken orally potentiates the action of a bronchodilator inhalant administered 90 minutes later, whereas no additive effect occurs when the drugs are given in reverse order. -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -

Clinical Trial of Orciprenaline in Bradyarrhythmias
100 SINGAPORE MEDICAL JOURNAL Vol. 16, No. 2. June, 1975. CLINICAL TRIAL OF ORCIPRENALINE IN BRADYARRHYTHMIAS By Chin Hock, Lirn, Charles C. S. Toh and Oon Teik, Khoo SYNOPSIS The intravenous and oral forms of orciprenaline was used in the treatment of patients with severe sinus bradycardia, high grade atrio -ventricular block or the sick sinus syndrome. Intra- venous orciprenaline can be used to tide patients over a period of severe bradyarrhythmia, or prior to cardiac pacing. One patient developed ventricular tachycardia following an intravenous bolus of orciprenaline. Oral orciprenaline was used in 23 patients with severe bradyarrhythmia. Two patients responded poorly to it. Saventrine was given sequentially in nine patients. The merits and side effects of both drugs are discussed. INTRODUCTION efficiency of orciprenaline and to compare it with Saventrine in the treatment of complete In 1952, Nathanson and Miller introduced heart block and other forms of bradyarrhyth- isoprenaline for the treatment of heart block and mias. it still has an important role in the treatment of bradyarrhythmias. Nis Innissen and Thompson PHARMACOLOGY (1965) felt that the use of a sustained release form of isoprenaline could reduce the need for Orciprenaline (Alupent*) is a derivative of pacemakers. Intravenous isoprenaline and orci- isoprenaline but it has predominant beta -two prenaline may be used to treat patients with stimulatine effects and is commonly used as a Stokes Adams (S.A.) attacks resulting from very bronchodilator (Bogdan, 1969). However, it has slow ventricular rates in acute myocardial chronotropic and inotropic effects. It stimulates infarction, either alone or prior to cardiac the sino -atrial and atrioventricular nodes and pacing. -

Orciprenaline Sulphate (Alupent): Planned Withdrawal from the UK Market Following a Risk-Benefit Analysis
MHRA PUBLIC ASSESSMENT REPORT Orciprenaline sulphate (Alupent): planned withdrawal from the UK market following a risk-benefit analysis November 2009 Executive summary 2 1. Introduction 3 2. Summary of data 3 2.1 Clinical pharmacology 3 2.2 Efficacy 3 2.3 Safety 4 3. Conclusions 8 4. References 9 5. Glossary 10 1 EXECUTIVE SUMMARY (Please note that this summary is intended to be accessible to all members of the public, including health professionals) Background The Medicines and Healthcare products Regulatory Agency (MHRA) is the government agency responsible for regulating the effectiveness and safety of medicines and medical devices in the UK. We continually review the safety of all medicines in the UK, and inform healthcare professionals and the public of the latest safety updates. In our Public Assessment Reports, we discuss the evidence for a safety issue with a particular drug or drug class, and changes made to the product information for the drug on the basis of this evidence, which will help safeguard public health. This MHRA Public Assessment Report discusses a review of the risks and benefits of a medicine called orciprenaline sulphate. Orciprenaline sulphate is available for oral administration as a syrup used to treat reversible airways obstructiona, which is a symptom of asthmab and chronic obstructive pulmonary diseasec. It acts on specific areas in the body called β- receptors, which relaxes the muscles used for breathing and opens the airways in the lungs. Orciprenaline sulphate was licensed in 1972 and is marketed in the UK under the brand name Alupent Syrup. As with any medicine, the use of orciprenaline sulphate may lead to adverse reactions (side-effects) in some individuals, which are described in the product information, including the patient information leaflet (see the Electronic Medicines Compendium (product information) website). -

UV-Vis-5-Compressed.Pdf
Ultraviolet and visible spectroscopy David G. Watson Lecture 5 EMADALSAMARRAI Fig.4.13 The complex formed between adrenaline and iron, which is used to analyse adrenaline at low levels in an injection. Adrenaline iron (ll) complex l'iiiitl.iiitiliiiil Self-test 4.7 iiri:lilllr:i!!!f !!:ill Adrenaline in bupivacaine/adrenaline injection is assayed by complex formation with iron (ll). 20 ml of the injection is mixed with 0.2 ml of reagent and 2 ml of buffer and a reading istaken in a 4 cm pathlength cell. A reading of a solution containing 5.21 pglml of adrenaline is taken under the same conditions. The following results were obtained: . Absorbance of sample:0.173 . Absorbance of standard solution:0.18i Calculate the percentage of w/v of adrenaline in the injection. t n/rvr %s000'0 :JaMsuv Difference spectrop hotometry In difference spectroscopy, a component in a mixture is analysed by carrying out a reaction which is selective for the analyte. This could be simply bringing about a shift in wavelength through adjustment of the pH of the solution in which the analyte is dissolved or a chemical reaction such as oxidation or reduction. In the following example the selective alkaline shift of aspirin is used to determine it in a preparation also containing dextropropoxyphene, naphthalene sulphonic acid and caffeine. Caffeine, dextropropoxyphene and the naphthalene sulphonic acid anion do not undergo appreciable alkaline shifts whereas aspirin does. Figure 4.14A shows the spectrum of the extract from tablets in 0.1 M HCI - in fact there is relatively minor interference at the wavelength used for the determination of aspirin but by using the sample in HCI in place of a blank in the reference cell one can be sure that interference from the excipients is eliminated. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau Et Al
US 20150202317A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau et al. (43) Pub. Date: Jul. 23, 2015 (54) DIPEPTDE-BASED PRODRUG LINKERS Publication Classification FOR ALPHATIC AMNE-CONTAINING DRUGS (51) Int. Cl. A647/48 (2006.01) (71) Applicant: Ascendis Pharma A/S, Hellerup (DK) A638/26 (2006.01) A6M5/9 (2006.01) (72) Inventors: Harald Rau, Heidelberg (DE); Torben A 6LX3/553 (2006.01) Le?mann, Neustadt an der Weinstrasse (52) U.S. Cl. (DE) CPC ......... A61K 47/48338 (2013.01); A61 K3I/553 (2013.01); A61 K38/26 (2013.01); A61 K (21) Appl. No.: 14/674,928 47/48215 (2013.01); A61M 5/19 (2013.01) (22) Filed: Mar. 31, 2015 (57) ABSTRACT The present invention relates to a prodrug or a pharmaceuti Related U.S. Application Data cally acceptable salt thereof, comprising a drug linker conju (63) Continuation of application No. 13/574,092, filed on gate D-L, wherein D being a biologically active moiety con Oct. 15, 2012, filed as application No. PCT/EP2011/ taining an aliphatic amine group is conjugated to one or more 050821 on Jan. 21, 2011. polymeric carriers via dipeptide-containing linkers L. Such carrier-linked prodrugs achieve drug releases with therapeu (30) Foreign Application Priority Data tically useful half-lives. The invention also relates to pharma ceutical compositions comprising said prodrugs and their use Jan. 22, 2010 (EP) ................................ 10 151564.1 as medicaments. US 2015/0202317 A1 Jul. 23, 2015 DIPEPTDE-BASED PRODRUG LINKERS 0007 Alternatively, the drugs may be conjugated to a car FOR ALPHATIC AMNE-CONTAINING rier through permanent covalent bonds. -

(12) United States Patent (10) Patent No.: US 9,376.418 B2 Haidle Et Al
USOO9376418B2 (12) United States Patent (10) Patent No.: US 9,376.418 B2 Haidle et al. (45) Date of Patent: Jun. 28, 2016 (54) SUBSTITUTED PYRIDINE SPLEEN 31/5377 (2013.01); A61K3I/5383 (2013.01); TYROSINE KINASE (SYK) INHIBITORS A61 K3I/551 (2013.01); A61K 45/06 (2013.01); C07D401/14 (2013.01); C07D (71) Applicants: Merck Sharp & Dohme Corp., 405/14 (2013.01); C07D 409/14 (2013.01); Rahway, NJ (US); Merck Canada Inc., C07D 413/14 (2013.01); C07D 417/14 Kirkland, Quebec (CA) (2013.01); C07D 471/04 (2013.01); (Continued) Inventors: (72) Andrew M. Haidle, Cambridge, MA (58) Field of Classification Search (US); Sandra Lee Knowles, Princeton, CPC ............... CO7D 401/12: CO7D 401/14: A61 K NJ (US); Solomon D. Kattar, Arlington, 31/5377; A61K31/506 MA (US); Denis Deschenes, Lachine USPC ........................ 544/122, 331; 514/235.8, 275 (CA); Jason Burch, Redwood City, CA See application file for complete search history. (US); Joel Robichaud, Dollard-des-Ormeaux (CA); Matthew (56) References Cited Christopher, Brookline, MA (US); Michael D. Altman, Needham, MA U.S. PATENT DOCUMENTS (US); James P. Jewell, Newtown, MA 5,710,129 A 1/1998 Lynch et al. (US); Alan B. Northrup, Reading, VA 6/2001 Uckun et al. (US); Marc Blouin, Saint-Lazare (CA); 6,248,790 B1 John Michael Ellis, Needham, MA (Continued) (US); Hua Zhou, Acton, MA (US); Christian Fischer, Natick, MA (US); FOREIGN PATENT DOCUMENTS Adam J. Schell, Decatur, GA (US); EP 877O2O A1 11, 1998 Michael H. Reutershan, Brookline, MA EP 2441755 A1 4/2012 (US); Brandon M. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -
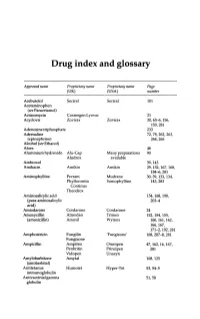
Drug Index and Glossary
Drug index and glossary Approved name Proprietary name Proprietary name Page (UK) (USA) number Acebutolol Sectral Sectral 101 Acetaminophen (see Paracetamol) Actinomycin Cosmegen Lyovac 21 Acyclovir Zovirax Zovirax 30, 65-6, 156, 159,281 Adenosine triphosphate 233 Adrenaline 72, 78, 262, 263, (epinephrine) 264,265 Alcohol (see Ethanol) Aloes 48 Aluminium hydroxide Alu-Cap Many preparations 90 Aludrox available Ambroxol 39,143 Amikacin Amikin Amikin 29,152,167,168, 184-6,281 Aminophylline Pecram Mudrane 30,39,133,134, Phyllocontin Somophylline 143,283 Continus Theodrox Aminosalicylic acid 154, 168, 198, (para-aminosalicylic 203-4 acid) Amiodarone Cordarone Cordarone 24 Amoxycillin Almodan Trlmox 152, 154, 155, (amoxicillin) Amoxil Wymox 160, 161, 162, 166,167, 171-2, 192, 281 Amphotericin Fungilin 'Fungizone' 168, 207--S, 281 Fungizone Ampicillin Ampifen Omnipen 47,162,16,167, Penbritin Principen 281 Vidopen Unasyn Amylobarbitone Amytal 108,125 (amobarbital) Antitetanus Humotet Hyper-Tet 53,54-5 immunoglobulin Antivaccinial gamma 51,58 globulin Drug index and glossary 289 Approved name Proprietary name Proprietary name Page (UK) (USA) number Antivaricella-zoster 65 immunoglobulin Apomorphine 97 Arnica 278 Ascorbic acid Redoxon Many preparations 70,75--6 available Aspirin Many preparations Many preparations 23,48,99,100, available available 141,272-3,276 Atebrin 12 (Atabrine, Medacrine, Quinacrine) Atenolol Tenormin Tenormin 101,120 Atropine Many combined Many combined 48,49,96,222, preparations preparations 234,250,26S-9 available available -

Pharmacology and Therapeutics of Bronchodilators
1521-0081/12/6403-450–504$25.00 PHARMACOLOGICAL REVIEWS Vol. 64, No. 3 Copyright © 2012 by The American Society for Pharmacology and Experimental Therapeutics 4580/3762238 Pharmacol Rev 64:450–504, 2012 ASSOCIATE EDITOR: DAVID R. SIBLEY Pharmacology and Therapeutics of Bronchodilators Mario Cazzola, Clive P. Page, Luigino Calzetta, and M. Gabriella Matera Department of Internal Medicine, Unit of Respiratory Clinical Pharmacology, University of Rome ‘Tor Vergata,’ Rome, Italy (M.C., L.C.); Department of Pulmonary Rehabilitation, San Raffaele Pisana Hospital, Istituto di Ricovero e Cura a Carattere Scientifico, Rome, Italy (M.C., L.C.); Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, London, UK (C.P.P., L.C.); and Department of Experimental Medicine, Unit of Pharmacology, Second University of Naples, Naples, Italy (M.G.M.) Abstract............................................................................... 451 I. Introduction: the physiological rationale for using bronchodilators .......................... 452 II. -Adrenergic receptor agonists .......................................................... 455 A. A history of the development of -adrenergic receptor agonists: from nonselective  Downloaded from adrenergic receptor agonists to 2-adrenergic receptor-selective drugs.................... 455  B. Short-acting 2-adrenergic receptor agonists........................................... 457 1. Albuterol........................................................................ 457