The Biodiversity and Metabolism of Peatland Pools
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Water Beetles
Ireland Red List No. 1 Water beetles Ireland Red List No. 1: Water beetles G.N. Foster1, B.H. Nelson2 & Á. O Connor3 1 3 Eglinton Terrace, Ayr KA7 1JJ 2 Department of Natural Sciences, National Museums Northern Ireland 3 National Parks & Wildlife Service, Department of Environment, Heritage & Local Government Citation: Foster, G. N., Nelson, B. H. & O Connor, Á. (2009) Ireland Red List No. 1 – Water beetles. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover images from top: Dryops similaris (© Roy Anderson); Gyrinus urinator, Hygrotus decoratus, Berosus signaticollis & Platambus maculatus (all © Jonty Denton) Ireland Red List Series Editors: N. Kingston & F. Marnell © National Parks and Wildlife Service 2009 ISSN 2009‐2016 Red list of Irish Water beetles 2009 ____________________________ CONTENTS ACKNOWLEDGEMENTS .................................................................................................................................... 1 EXECUTIVE SUMMARY...................................................................................................................................... 2 INTRODUCTION................................................................................................................................................ 3 NOMENCLATURE AND THE IRISH CHECKLIST................................................................................................ 3 COVERAGE ....................................................................................................................................................... -

CHIRONOMUS Newsletter on Chironomidae Research
CHIRONOMUS Newsletter on Chironomidae Research No. 25 ISSN 0172-1941 (printed) 1891-5426 (online) November 2012 CONTENTS Editorial: Inventories - What are they good for? 3 Dr. William P. Coffman: Celebrating 50 years of research on Chironomidae 4 Dear Sepp! 9 Dr. Marta Margreiter-Kownacka 14 Current Research Sharma, S. et al. Chironomidae (Diptera) in the Himalayan Lakes - A study of sub- fossil assemblages in the sediments of two high altitude lakes from Nepal 15 Krosch, M. et al. Non-destructive DNA extraction from Chironomidae, including fragile pupal exuviae, extends analysable collections and enhances vouchering 22 Martin, J. Kiefferulus barbitarsis (Kieffer, 1911) and Kiefferulus tainanus (Kieffer, 1912) are distinct species 28 Short Communications An easy to make and simple designed rearing apparatus for Chironomidae 33 Some proposed emendations to larval morphology terminology 35 Chironomids in Quaternary permafrost deposits in the Siberian Arctic 39 New books, resources and announcements 43 Finnish Chironomidae 47 Chironomini indet. (Paratendipes?) from La Selva Biological Station, Costa Rica. Photo by Carlos de la Rosa. CHIRONOMUS Newsletter on Chironomidae Research Editors Torbjørn EKREM, Museum of Natural History and Archaeology, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway Peter H. LANGTON, 16, Irish Society Court, Coleraine, Co. Londonderry, Northern Ireland BT52 1GX The CHIRONOMUS Newsletter on Chironomidae Research is devoted to all aspects of chironomid research and aims to be an updated news bulletin for the Chironomidae research community. The newsletter is published yearly in October/November, is open access, and can be downloaded free from this website: http:// www.ntnu.no/ojs/index.php/chironomus. Publisher is the Museum of Natural History and Archaeology at the Norwegian University of Science and Technology in Trondheim, Norway. -

Dytiscid Water Beetles (Coleoptera: Dytiscidae) of the Yukon
Dytiscid water beetles of the Yukon FRONTISPIECE. Neoscutopterus horni (Crotch), a large, black species of dytiscid beetle that is common in sphagnum bog pools throughout the Yukon Territory. 491 Dytiscid Water Beetles (Coleoptera: Dytiscidae) of the Yukon DAVID J. LARSON Department of Biology, Memorial University of Newfoundland St. John’s, Newfoundland, Canada A1B 3X9 Abstract. One hundred and thirteen species of Dytiscidae (Coleoptera) are recorded from the Yukon Territory. The Yukon distribution, total geographical range and habitat of each of these species is described and multi-species patterns are summarized in tabular form. Several different range patterns are recognized with most species being Holarctic or transcontinental Nearctic boreal (73%) in lentic habitats. Other major range patterns are Arctic (20 species) and Cordilleran (12 species), while a few species are considered to have Grassland (7), Deciduous forest (2) or Southern (5) distributions. Sixteen species have a Beringian and glaciated western Nearctic distribution, i.e. the only Nearctic Wisconsinan refugial area encompassed by their present range is the Alaskan/Central Yukon refugium; 5 of these species are closely confined to this area while 11 have wide ranges that extend in the arctic and/or boreal zones east to Hudson Bay. Résumé. Les dytiques (Coleoptera: Dytiscidae) du Yukon. Cent treize espèces de dytiques (Coleoptera: Dytiscidae) sont connues au Yukon. Leur répartition au Yukon, leur répartition globale et leur habitat sont décrits et un tableau résume les regroupements d’espèces. La répartition permet de reconnaître plusieurs éléments: la majorité des espèces sont holarctiques ou transcontinentales-néarctiques-boréales (73%) dans des habitats lénitiques. Vingt espèces sont arctiques, 12 sont cordillériennes, alors qu’un petit nombre sont de la prairie herbeuse (7), ou de la forêt décidue (2), ou sont australes (5). -

Checklist of the Family Chironomidae (Diptera) of Finland
A peer-reviewed open-access journal ZooKeys 441: 63–90 (2014)Checklist of the family Chironomidae (Diptera) of Finland 63 doi: 10.3897/zookeys.441.7461 CHECKLIST www.zookeys.org Launched to accelerate biodiversity research Checklist of the family Chironomidae (Diptera) of Finland Lauri Paasivirta1 1 Ruuhikoskenkatu 17 B 5, FI-24240 Salo, Finland Corresponding author: Lauri Paasivirta ([email protected]) Academic editor: J. Kahanpää | Received 10 March 2014 | Accepted 26 August 2014 | Published 19 September 2014 http://zoobank.org/F3343ED1-AE2C-43B4-9BA1-029B5EC32763 Citation: Paasivirta L (2014) Checklist of the family Chironomidae (Diptera) of Finland. In: Kahanpää J, Salmela J (Eds) Checklist of the Diptera of Finland. ZooKeys 441: 63–90. doi: 10.3897/zookeys.441.7461 Abstract A checklist of the family Chironomidae (Diptera) recorded from Finland is presented. Keywords Finland, Chironomidae, species list, biodiversity, faunistics Introduction There are supposedly at least 15 000 species of chironomid midges in the world (Armitage et al. 1995, but see Pape et al. 2011) making it the largest family among the aquatic insects. The European chironomid fauna consists of 1262 species (Sæther and Spies 2013). In Finland, 780 species can be found, of which 37 are still undescribed (Paasivirta 2012). The species checklist written by B. Lindeberg on 23.10.1979 (Hackman 1980) included 409 chironomid species. Twenty of those species have been removed from the checklist due to various reasons. The total number of species increased in the 1980s to 570, mainly due to the identification work by me and J. Tuiskunen (Bergman and Jansson 1983, Tuiskunen and Lindeberg 1986). -

The Formation of Water Beetle Fauna in Anthropogenic Water Bodies
Oceanological and Hydrobiological Studies International Journal of Oceanography and Hydrobiology Vol. XXXVII, No. 1 Institute of Oceanography (31-42) University of Gdańsk ISSN 1730-413X 2008 eISSN 1897-3191 Received: July 03, 2007 DOI 10.2478/v10009-007-0037-y Original research paper Accepted: January 17, 2008 The formation of water beetle fauna in anthropogenic water bodies Joanna Pakulnicka1 Department of Ecology and Environmental Protection University of Warmia and Mazury Plac Łódzki 3, 10–727 Olsztyn Poland Key words: water beetles, clay-pits, gravel-pits, succession, Poland Abstract Studies on the fauna of water beetles inhabiting anthropogenic water bodies were conducted on 44 clay-pit and gravel-pit ponds. A total of 125 water beetle species were identified. The dominant species were Scarodytes halensis and Laccobius minutus, representing the argillophilous component. Eurytopic, lake and riverine, and peatland species were also identified. Among the environmental factors determining the diversity of the water beetle fauna in particular types of habitats, the most significant role was played by the substratum and succession stage. 1 e-mail: [email protected] Copyright© by Institute of Oceanography, University of Gdańsk, Poland www.oandhs.org 32 J. Pakulnicka INTRODUCTION Anthropogenic water bodies, formed as a result of mineral extraction, are an important element of a hydrographical network. However, they have not aroused much interest among hydrobiologists. Polish literature on aquatic Coleoptera provides scant information on artificial reservoirs, although the ecology of water beetles is relatively well-known. Noteworthy works devoted to this subject include those by Łęgosz-Owsianna (1955), Tranda (1959), Biesiadka (1977), Mielewczyk (1997, 1997a) as well as by Kowalik and Buczyński (2003). -

Ana Kurbalija PREGLED ENTOMOFAUNE MOČVARNIH
SVEUČILIŠTE JOSIPA JURJA STROSSMAYERA U OSIJEKU I INSTITUT RUĐER BOŠKOVI Ć, ZAGREB Poslijediplomski sveučilišni interdisciplinarni specijalisti čki studij ZAŠTITA PRIRODE I OKOLIŠA Ana Kurbalija PREGLED ENTOMOFAUNE MOČVARNIH STANIŠTA OD MEĐUNARODNOG ZNAČENJA U REPUBLICI HRVATSKOJ Specijalistički rad Osijek, 2012. TEMELJNA DOKUMENTACIJSKA KARTICA Sveučilište Josipa Jurja Strossmayera u Osijeku Specijalistički rad Institit Ruđer Boškovi ć, Zagreb Poslijediplomski sveučilišni interdisciplinarni specijalisti čki studij zaštita prirode i okoliša Znanstveno područje: Prirodne znanosti Znanstveno polje: Biologija PREGLED ENTOMOFAUNE MOČVARNIH STANIŠTA OD ME ĐUNARODNOG ZNAČENJA U REPUBLICI HRVATSKOJ Ana Kurbalija Rad je izrađen na Odjelu za biologiju, Sveučilišta Josipa Jurja Strossmayera u Osijeku Mentor: izv.prof. dr. sc. Stjepan Krčmar U ovom radu je istražen kvalitativni sastav entomof aune na četiri močvarna staništa od me đunarodnog značenja u Republici Hrvatskoj. To su Park prirode Kopački rit, Park prirode Lonjsko polje, Delta rijeke Neretve i Crna Mlaka. Glavni cilj specijalističkog rada je objediniti sve objavljene i neobjavljene podatke o nalazima vrsta kukaca na ova četiri močvarna staništa te kvalitativno usporediti entomofau nu pomoću Sörensonovog indexa faunističke sličnosti. Na području Parka prirode Kopački rit utvrđeno je ukupno 866 vrsta kukaca razvrstanih u 84 porodice i 513 rodova. Na području Parka prirode Lonjsko polje utvrđeno je 513 vrsta kukaca razvrstanih u 24 porodice i 89 rodova. Na području delte rijeke Neretve utvrđeno je ukupno 348 vrsta kukaca razvrstanih u 89 porodica i 227 rodova. Za područje Crne Mlake nije bilo dostupne literature o nalazima kukaca. Velika vrijednost Sörensonovog indexa od 80,85% ukazuje na veliku faunističku sličnost između faune obada Kopačkoga rita i Lonjskoga polja. Najmanja sličnost u fauni obada utvrđena je između močvarnih staništa Lonjskog polja i delte rijeke Neretve, a iznosi 41,37%. -

DNA Barcoding
Full-time PhD studies of Ecology and Environmental Protection Piotr Gadawski Species diversity and origin of non-biting midges (Chironomidae) from a geologically young lake PhD Thesis and its old spring system Performed in Department of Invertebrate Zoology and Hydrobiology in Institute of Ecology and Environmental Protection Różnorodność gatunkowa i pochodzenie fauny Supervisor: ochotkowatych (Chironomidae) z geologicznie Prof. dr hab. Michał Grabowski młodego jeziora i starego systemu źródlisk Auxiliary supervisor: Dr. Matteo Montagna, Assoc. Prof. Łódź, 2020 Łódź, 2020 Table of contents Acknowledgements ..........................................................................................................3 Summary ...........................................................................................................................4 General introduction .........................................................................................................6 Skadar Lake ...................................................................................................................7 Chironomidae ..............................................................................................................10 Species concept and integrative taxonomy .................................................................12 DNA barcoding ...........................................................................................................14 Chapter I. First insight into the diversity and ecology of non-biting midges (Diptera: Chironomidae) -
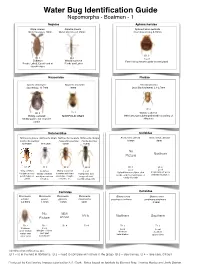
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Variations on a Theme
HENRY JOUTSIJOKI Variations on a Theme The Classification of Benthic Macroinvertebrates ACADEMIC DISSERTATION To be presented, with the permission of the board of the School of Information Sciences of the University of Tampere, for public discussion in the Auditorium Pinni B 1100, Kanslerinrinne 1, Tampere, on November 9th, 2012, at 12 o’clock. UNIVERSITY OF TAMPERE ACADEMIC DISSERTATION University of Tampere School of Information Sciences Finland Copyright ©2012 Tampere University Press and the author Distribution Tel. +358 40 190 9800 Bookshop TAJU [email protected] P.O. Box 617 www.uta.fi/taju 33014 University of Tampere http://granum.uta.fi Finland Cover design by Mikko Reinikka Acta Universitatis Tamperensis 1777 Acta Electronica Universitatis Tamperensis 1251 ISBN 978-951-44-8952-5 (print) ISBN 978-951-44-8953-2 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://acta.uta.fi Tampereen Yliopistopaino Oy – Juvenes Print Tampere 2012 Abstract This thesis focused on the classification of benthic macroinvertebrates by us- ing machine learning methods. Special emphasis was placed on multi-class extensions of Support Vector Machines (SVMs). Benthic macroinvertebrates are used in biomonitoring due to their properties to react to changes in water quality. The use of benthic macroinvertebrates in biomonitoring requires a large number of collected samples. Traditionally benthic macroinvertebrates are separated and identified manually one by one from samples collected by biologists. This, however, is a time-consuming and expensive approach. By the automation of the identification process time and money would be saved and more extensive biomonitoring would be possible. The aim of the thesis was to examine what classification method would be the most appro- priate for automated benthic macroinvertebrate classification. -

A Review of the Hemiptera of Great Britain: the Aquatic and Semi-Aquatic Bugs
Natural England Commissioned Report NECR188 A review of the Hemiptera of Great Britain: The Aquatic and Semi-aquatic Bugs Dipsocoromorpha, Gerromorpha, Leptopodomorpha & Nepomorpha Species Status No.24 First published 20 November 2015 www.gov.uk/natural -england Foreword Natural England commission a range of reports from external contractors to provide evidence and advice to assist us in delivering our duties. The views in this report are those of the authors and do not necessarily represent those of Natural England. Background Making good decisions to conserve species should primarily be based upon an objective process of determining the degree of threat to the survival of a species. The recognised international approach to undertaking this is by assigning the species to one of the IUCN threat categories. This report was commissioned to update the national status of aquatic and semi-aquatic bugs using IUCN methodology for assessing threat. It covers all species of aquatic and semi-aquatic bugs (Heteroptera) in Great Britain, identifying those that are rare and/or under threat as well as non-threatened and non-native species. Reviews for other invertebrate groups will follow. Natural England Project Manager - Jon Webb, [email protected] Contractor - A.A. Cook (author) Keywords - invertebrates, red list, IUCN, status reviews, Heteroptera, aquatic bugs, shore bugs, IUCN threat categories, GB rarity status Further information This report can be downloaded from the Natural England website: www.gov.uk/government/organisations/natural-england. For information on Natural England publications contact the Natural England Enquiry Service on 0845 600 3078 or e-mail [email protected]. -

“When We Went Onto the Land, the Elders Taught Me to Not Drink from a Lake If There Were No Insects, Because That Lake Was Dead.”
“When we went onto the land, the elders taught me to not drink from a lake if there were no insects, because that lake was dead.” - Inuvialuit community member, Personal communication, 2017 THE IMPACTS OF RECENT WILDFIRES ON STREAM WATER QUALITY AND MACROINVERTEBRATE ASSEMBLAGES IN SOUTHERN NORTHWEST TERRITORIES, CANADA By Caitlin Sarah Garner, B.Sc. (Hons.) A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements of the degree of Master of Sustainability Brock University St. Catharines, ON ¬2017 Caitlin S. Garner Abstract High-latitude regions are currently undergoing rapid ecosystem change due to increasing temperatures and modified precipitation regimes. Since 2012, the Northwest Territories (Canada) has been experiencing severe drought and wildfire seasons. In 2014 alone, fires within the Northwest Territories consumed over 3.4 million hectares of forested land; 1.4 times larger than the national yearly average for Canada. Wildfire is one of the most important agents influencing age structure and composition of forest stands, as such, it is a critical factor in ecosystem dynamics. The impacts of wildfire on terrestrial systems garner more attention compared to aquatic habitats. This is especially true when considering aquatic ecosystems, specifically sub-arctic streams, where the impact of fires on stream ecology and chemistry are relatively understudied. Freshwater ecosystems, such as lakes and streams, are relied upon by northern communities for their cultural significance and economic and environmental goods and services they produce, including country foods. This study examines the impact of recent wildfire on freshwater streams within the North Slave, South Slave, and Dehcho regions of the Northwest Territories (Canada) through analysis of their water chemistry and benthic macroinvertebrate assemblages. -

The Role of Chironomidae in Separating Naturally Poor from Disturbed Communities
From taxonomy to multiple-trait bioassessment: the role of Chironomidae in separating naturally poor from disturbed communities Da taxonomia à abordagem baseada nos multiatributos dos taxa: função dos Chironomidae na separação de comunidades naturalmente pobres das antropogenicamente perturbadas Sónia Raquel Quinás Serra Tese de doutoramento em Biociências, ramo de especialização Ecologia de Bacias Hidrográficas, orientada pela Doutora Maria João Feio, pelo Doutor Manuel Augusto Simões Graça e pelo Doutor Sylvain Dolédec e apresentada ao Departamento de Ciências da Vida da Faculdade de Ciências e Tecnologia da Universidade de Coimbra. Agosto de 2016 This thesis was made under the Agreement for joint supervision of doctoral studies leading to the award of a dual doctoral degree. This agreement was celebrated between partner institutions from two countries (Portugal and France) and the Ph.D. student. The two Universities involved were: And This thesis was supported by: Portuguese Foundation for Science and Technology (FCT), financing program: ‘Programa Operacional Potencial Humano/Fundo Social Europeu’ (POPH/FSE): through an individual scholarship for the PhD student with reference: SFRH/BD/80188/2011 And MARE-UC – Marine and Environmental Sciences Centre. University of Coimbra, Portugal: CNRS, UMR 5023 - LEHNA, Laboratoire d'Ecologie des Hydrosystèmes Naturels et Anthropisés, University Lyon1, France: Aos meus amados pais, sempre os melhores e mais dedicados amigos Table of contents: ABSTRACT .....................................................................................................................