Gene Section Mini Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Defining Functional Interactions During Biogenesis of Epithelial Junctions
ARTICLE Received 11 Dec 2015 | Accepted 13 Oct 2016 | Published 6 Dec 2016 | Updated 5 Jan 2017 DOI: 10.1038/ncomms13542 OPEN Defining functional interactions during biogenesis of epithelial junctions J.C. Erasmus1,*, S. Bruche1,*,w, L. Pizarro1,2,*, N. Maimari1,3,*, T. Poggioli1,w, C. Tomlinson4,J.Lees5, I. Zalivina1,w, A. Wheeler1,w, A. Alberts6, A. Russo2 & V.M.M. Braga1 In spite of extensive recent progress, a comprehensive understanding of how actin cytoskeleton remodelling supports stable junctions remains to be established. Here we design a platform that integrates actin functions with optimized phenotypic clustering and identify new cytoskeletal proteins, their functional hierarchy and pathways that modulate E-cadherin adhesion. Depletion of EEF1A, an actin bundling protein, increases E-cadherin levels at junctions without a corresponding reinforcement of cell–cell contacts. This unexpected result reflects a more dynamic and mobile junctional actin in EEF1A-depleted cells. A partner for EEF1A in cadherin contact maintenance is the formin DIAPH2, which interacts with EEF1A. In contrast, depletion of either the endocytic regulator TRIP10 or the Rho GTPase activator VAV2 reduces E-cadherin levels at junctions. TRIP10 binds to and requires VAV2 function for its junctional localization. Overall, we present new conceptual insights on junction stabilization, which integrate known and novel pathways with impact for epithelial morphogenesis, homeostasis and diseases. 1 National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. 2 Computing Department, Imperial College London, London SW7 2AZ, UK. 3 Bioengineering Department, Faculty of Engineering, Imperial College London, London SW7 2AZ, UK. 4 Department of Surgery & Cancer, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. -

Bioinformatic Analysis of Structure and Function of LIM Domains of Human Zyxin Family Proteins
International Journal of Molecular Sciences Article Bioinformatic Analysis of Structure and Function of LIM Domains of Human Zyxin Family Proteins M. Quadir Siddiqui 1,† , Maulik D. Badmalia 1,† and Trushar R. Patel 1,2,3,* 1 Alberta RNA Research and Training Institute, Department of Chemistry and Biochemistry, University of Lethbridge, 4401 University Drive, Lethbridge, AB T1K 3M4, Canada; [email protected] (M.Q.S.); [email protected] (M.D.B.) 2 Department of Microbiology, Immunology and Infectious Disease, Cumming School of Medicine, University of Calgary, 3330 Hospital Drive, Calgary, AB T2N 4N1, Canada 3 Li Ka Shing Institute of Virology, University of Alberta, Edmonton, AB T6G 2E1, Canada * Correspondence: [email protected] † These authors contributed equally to the work. Abstract: Members of the human Zyxin family are LIM domain-containing proteins that perform critical cellular functions and are indispensable for cellular integrity. Despite their importance, not much is known about their structure, functions, interactions and dynamics. To provide insights into these, we used a set of in-silico tools and databases and analyzed their amino acid sequence, phylogeny, post-translational modifications, structure-dynamics, molecular interactions, and func- tions. Our analysis revealed that zyxin members are ohnologs. Presence of a conserved nuclear export signal composed of LxxLxL/LxxxLxL consensus sequence, as well as a possible nuclear localization signal, suggesting that Zyxin family members may have nuclear and cytoplasmic roles. The molecular modeling and structural analysis indicated that Zyxin family LIM domains share Citation: Siddiqui, M.Q.; Badmalia, similarities with transcriptional regulators and have positively charged electrostatic patches, which M.D.; Patel, T.R. -

Alpha;-Actinin-4 Promotes Metastasis in Gastric Cancer
Laboratory Investigation (2017) 97, 1084–1094 © 2017 USCAP, Inc All rights reserved 0023-6837/17 α-Actinin-4 promotes metastasis in gastric cancer Xin Liu and Kent-Man Chu Metastasis increases the mortality rate of gastric cancer, which is the third leading cause of cancer-associated deaths worldwide. This study aims to identify the genes promoting metastasis of gastric cancer (GC). A human cell motility PCR array was used to analyze a pair of tumor and non-tumor tissue samples from a patient with stage IV GC (T3N3M1). Expression of the dysregulated genes was then evaluated in GC tissue samples (n = 10) and cell lines (n =6) via qPCR. Expression of α-actinin-4 (ACTN4) was validated in a larger sample size (n = 47) by qPCR, western blot and immunohistochemistry. Knockdown of ACTN4 with specific siRNAs was performed in GC cells, and adhesion assays, transwell invasion assays and migration assays were used to evaluate the function of these cells. Expression of potential targets of ACTN4 were then evaluated by qPCR. Thirty upregulated genes (greater than twofold) were revealed by the PCR array. We focused on ACTN4 because it was upregulated in 6 out of 10 pairs of tissue samples and 5 out of 6 GC cell lines. Further study indicated that ACTN4 was upregulated in 22/32 pairs of tissue samples at stage III & IV (P = 0.0069). Knockdown of ACTN4 in GC cells showed no significant effect on cell proliferation, but significantly increased cell-matrix adhesion, as well as reduced migration and invasion of AGS, MKN7 and NCI-N87 cells. -

Atlas Journal
Atlas of Genetics and Cytogenetics in Oncology and Haematology Home Genes Leukemias Tumors Cancer prone Deep Insight Case Reports Portal Journals Teaching X Y 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 NA Atlas Journal Atlas Journal versus Atlas Database: the accumulation of the issues of the Journal constitutes the body of the Database/Text-Book. TABLE OF CONTENTS Volume 13, Number 7, July 2009 Previous Issue / Next Issue Genes ABL1 (v-abl Abelson murine leukemia viral oncogene homolog 1) (9q34.1) - updated. Ali G Turhan. Atlas Genet Cytogenet Oncol Haematol 2009; 13 (7): 757-766. [Full Text] [PDF] URL : http://atlasgeneticsoncology.org/Genes/ABL.html BCL2L12 (BCL2-like 12 (proline-rich)) (19q13.3). Christos Kontos, Hellinida Thomadaki, Andreas Scorilas. Atlas Genet Cytogenet Oncol Haematol 2009; 13 (7): 767-771. [Full Text] [PDF] URL : http://atlasgeneticsoncology.org/Genes/BCL2L12ID773ch19q13.html BCR (Breakpoint cluster region) (22q11.2) - updated. Ali G Turhan. Atlas Genet Cytogenet Oncol Haematol 2009; 13 (7): 772-779. [Full Text] [PDF] URL : http://atlasgeneticsoncology.org/Genes/BCR.html ENAH (enabled homolog (Drosophila)) (1q42.12). Paola Nisticò, Francesca Di Modugno. Atlas Genet Cytogenet Oncol Haematol 2009; 13 (7): 780-785. [Full Text] [PDF] URL : http://atlasgeneticsoncology.org/Genes/ENAHID44148ch1q42.html FGFR2 (fibroblast growth factor receptor 2) (10q26.13). Masaru Katoh. Atlas Genet Cytogenet Oncol Haematol 2009; 13 (7): 786-799. [Full Text] [PDF] URL : http://atlasgeneticsoncology.org/Genes/FGFR2ID40570ch10q26.html MAPK6 (mitogen-activated protein kinase 6) (15q21.2). Sylvain Meloche. Atlas Genet Cytogenet Oncol Haematol 2009; 13 (7): 800-804. -

Role and Regulation of the P53-Homolog P73 in the Transformation of Normal Human Fibroblasts
Role and regulation of the p53-homolog p73 in the transformation of normal human fibroblasts Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Bayerischen Julius-Maximilians-Universität Würzburg vorgelegt von Lars Hofmann aus Aschaffenburg Würzburg 2007 Eingereicht am Mitglieder der Promotionskommission: Vorsitzender: Prof. Dr. Dr. Martin J. Müller Gutachter: Prof. Dr. Michael P. Schön Gutachter : Prof. Dr. Georg Krohne Tag des Promotionskolloquiums: Doktorurkunde ausgehändigt am Erklärung Hiermit erkläre ich, dass ich die vorliegende Arbeit selbständig angefertigt und keine anderen als die angegebenen Hilfsmittel und Quellen verwendet habe. Diese Arbeit wurde weder in gleicher noch in ähnlicher Form in einem anderen Prüfungsverfahren vorgelegt. Ich habe früher, außer den mit dem Zulassungsgesuch urkundlichen Graden, keine weiteren akademischen Grade erworben und zu erwerben gesucht. Würzburg, Lars Hofmann Content SUMMARY ................................................................................................................ IV ZUSAMMENFASSUNG ............................................................................................. V 1. INTRODUCTION ................................................................................................. 1 1.1. Molecular basics of cancer .......................................................................................... 1 1.2. Early research on tumorigenesis ................................................................................. 3 1.3. Developing -

Downregulation of Glial Genes Involved in Synaptic Function
RESEARCH ARTICLE Downregulation of glial genes involved in synaptic function mitigates Huntington’s disease pathogenesis Tarik Seref Onur1,2,3†, Andrew Laitman2,4,5†, He Zhao2, Ryan Keyho2, Hyemin Kim2, Jennifer Wang2, Megan Mair1,2,3, Huilan Wang6, Lifang Li1,2, Alma Perez2, Maria de Haro1,2, Ying-Wooi Wan2, Genevera Allen2,7, Boxun Lu6, Ismael Al-Ramahi1,2, Zhandong Liu2,4,5, Juan Botas1,2,3,4* 1Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, United States; 2Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, Houston, United States; 3Genetics & Genomics Graduate Program, Baylor College of Medicine, Houston, United States; 4Quantitative & Computational Biosciences, Baylor College of Medicine, Houston, United States; 5Department of Pediatrics, Baylor College of Medicine, Houston, United States; 6State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Fudan University, Shanghai, China; 7Departments of Electrical & Computer Engineering, Statistics and Computer Science, Rice University, Houston, United States Abstract Most research on neurodegenerative diseases has focused on neurons, yet glia help form and maintain the synapses whose loss is so prominent in these conditions. To investigate the contributions of glia to Huntington’s disease (HD), we profiled the gene expression alterations of *For correspondence: Drosophila expressing human mutant Huntingtin (mHTT) in either glia or neurons and compared [email protected] these changes to what is observed in HD human and HD mice striata. A large portion of conserved genes are concordantly dysregulated across the three species; we tested these genes in a high- †These authors contributed throughput behavioral assay and found that downregulation of genes involved in synapse assembly equally to this work mitigated pathogenesis and behavioral deficits. -

Effect of Low Doses of Estradiol and Tamoxifen on Breast Cancer Cell Karyotypes
238 M Rondón-Lagos et al. Breast cancer cell karyotypes, 23:8 635–650 Research E2 and tamoxifen Effect of low doses of estradiol and tamoxifen on breast cancer cell karyotypes Milena Rondón-Lagos1, Nelson Rangel1,2, Ludovica Verdun Di Cantogno3, Laura Annaratone1, Isabella Castellano1, Rosalia Russo1, Tilde Manetta4, Caterina Marchiò1,* and Anna Sapino1,5,* 1Department of Medical Sciences, University of Turin, Turin, Italy Correspondence 2Natural and Mathematical Sciences Faculty, Universidad del Rosario, Bogotá, Colombia should be addressed 3Pathology Division, Azienda Ospedaliera Città della Salute e della Scienza di Torino, Turin, Italy to C Marchiò or A Sapino 4Department of Public Health and Pediatrics, University of Turin, Turin, Italy Email 5Candiolo Cancer Institute, FPO-IRCCS, Candiolo, Italy [email protected] or *(C Marchiò and A Sapino contributed equally to this work) [email protected] Abstract Evidence supports a role of 17β-estradiol (E2) in carcinogenesis and the large majority Key Words of breast carcinomas are dependent on estrogen. The anti-estrogen tamoxifen (TAM) f breast cancer cells is widely used for both treatment and prevention of breast cancer; however, it is also f estradiol carcinogenic in human uterus and rat liver, highlighting the profound complexity of its f tamoxifen actions. The nature of E2- or TAM-induced chromosomal damage has been explored using f chromosomal relatively high concentrations of these agents, and only some numerical aberrations abnormalities Endocrine-Related Cancer Endocrine-Related and chromosomal breaks have been analyzed. This study aimed to determine the effects f chromosomal instability −8 −1 −6 −1 of low doses of E2 and TAM (10 mol L and 10 mol L respectively) on karyotypes of MCF7, T47D, BT474, and SKBR3 breast cancer cells by comparing the results of conventional karyotyping and multi-FISH painting with cell proliferation. -

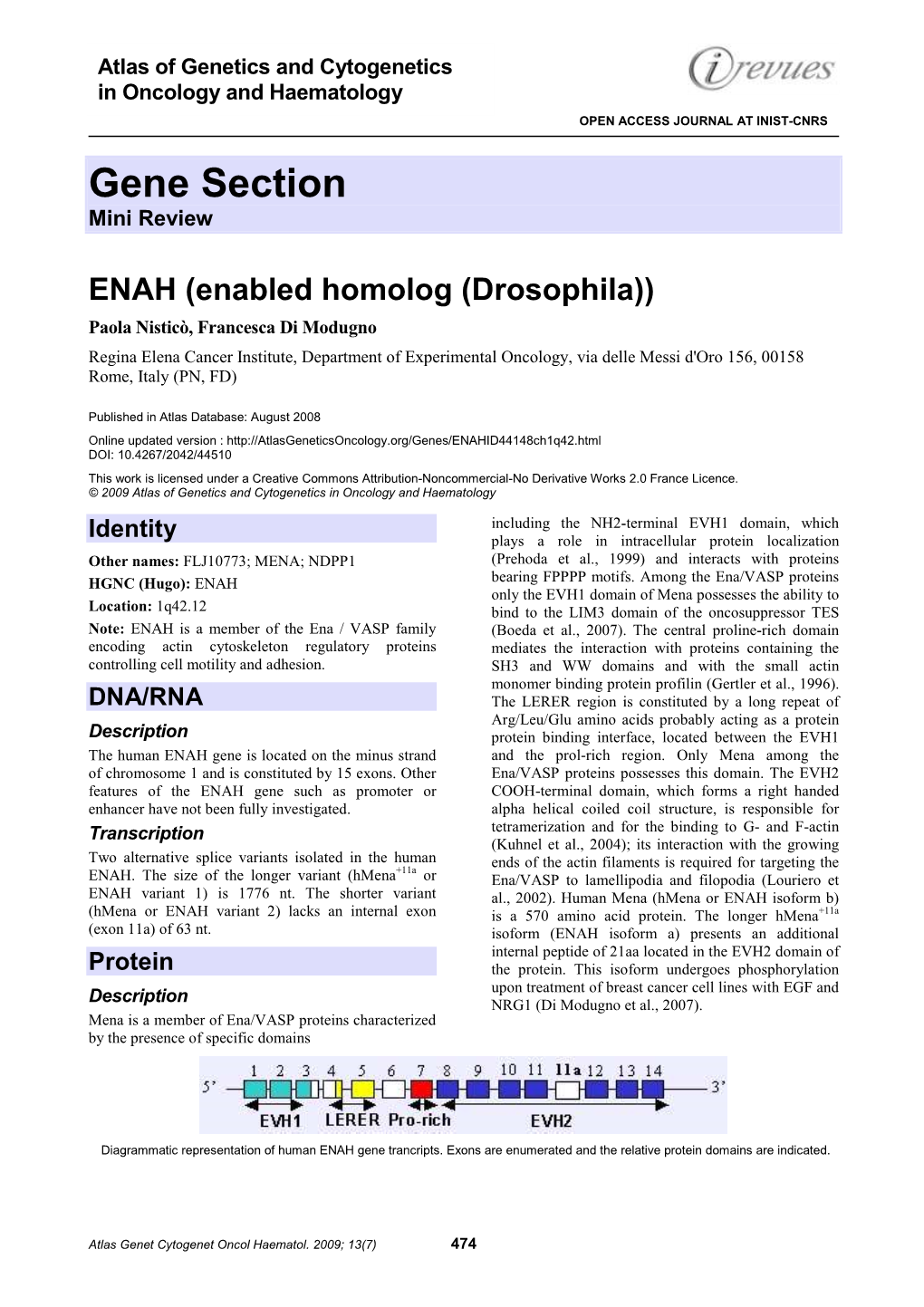
Molecular Cloning of Hmena (ENAH)
Research Article Molecular Cloning of hMena (ENAH) and Its Splice Variant hMena+11a: Epidermal Growth Factor Increases Their Expression and Stimulates hMena+11a Phosphorylation in Breast Cancer Cell Lines Francesca Di Modugno,1 Lucia DeMonte,5,6 Michele Balsamo,2 Giovanna Bronzi,2 Maria Rita Nicotra,3 Massimo Alessio,6 Elke Jager,7 John S. Condeelis,8 Angela Santoni,4 Pier Giorgio Natali,2 and Paola Nistico`2 1Experimental Chemotherapy and 2Laboratory of Immunology, Regina Elena Cancer Institute; 3Molecular Biology and Pathology Institute, National Research Council; 4Experimental Medicine and Pathology, University ‘‘La Sapienza,’’ Rome, Italy; 5Tumor Immunology and 6Proteome Biochemistry, Dibit, San Raffaele Scientific Institute, Milan, Italy; 7Medizinische Klinik II, Hamatologie-Onkologie, Krankenhaus Nordwest, Frankfurt, Germany; and 8Department of Anatomy, Structural Biology and Analytical Imaging Facility, Albert Einstein College of Medicine, Bronx, New York Abstract (serologic analysis of cDNA expression libraries) technology the hMena (ENAH), an actin regulatory protein involved in the hMena protein (3), the human orthologue of murine Mena, which control of cell motility and adhesion, is modulated during is overexpressed in benign breast lesions with high risk of human breast carcinogenesis. In fact, whereas undetectable in transformation and in >70% of primary breast cancers (4). normal mammary epithelium, hMena becomes overexpressed Mena belongs to the Ena/VASP protein family, which includes key regulatory molecules controlling cell shape (5, 6) and movement (7) in high-risk benign lesions and primary and metastatic tumors. + À by protecting actin filaments from capping proteins at their barbed In vivo, hMena overexpression correlates with the HER-2 /ER / + ends (8). Ena/VASP proteins are constituents of the adherens Ki67 unfavorable prognostic phenotype. -

Native Proline-Rich Motifs Exploit Sequence Context to Target Actin-Remodeling Ena/VASP Proteins
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.22.436451; this version posted June 18, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Native proline-rich motifs exploit sequence context to target actin-remodeling Ena/VASP proteins Theresa Hwang1, Robert A. Grant1, Meucci W. Ilunga1, Venkatesh Sivaraman1, and Amy E. Keating1,2* 1. Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 2. Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA *correspondence to [email protected] Abstract The human proteome is replete with short linear motifs (SLiMs) of 4-6 residues that are critical for protein-protein interactions, yet the importance of the sequence surrounding such motifs is underexplored. We devised a proteomic screen to systematically examine the influence of SLiM sequence context on protein-protein interactions. Focusing on the EVH1 domain of ENAH, an actin regulator that is upregulated in invasive cancers, we screened 36-residue proteome-derived peptides for binding. We discovered a pocket on the ENAH EVH1 domain that diverged from its orthologs to recognize extended SLiMs, and we found that proteins with two EVH1-binding SLiMs can wrap around a single domain. We also found that the ciliary protein PCARE uses an extended 23-residue region to obtain higher affinity than any known ENAH EVH1-binding motif. Our screen provides a way to uncover the effects of broader proteomic context on motif-mediated interactions, revealing diverse mechanisms of contextual control over EVH1 interactions and establishing that SLiMs can’t be fully understood outside of their native context. -

Indoxyl Sulfate and P-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance
BASIC RESEARCH www.jasn.org Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance Britt Opdebeeck ,1 Stuart Maudsley,2,3 Abdelkrim Azmi,3 Annelies De Maré,1 Wout De Leger,4 Bjorn Meijers,5,6 Anja Verhulst,1 Pieter Evenepoel,5,6 Patrick C. D’Haese,1 and Ellen Neven1 1Laboratory of Pathophysiology, Department of Biomedical Sciences, 2Receptor Biology Lab, Department of Biomedical Sciences, and 3Translational Neurobiology Group, Flanders Institute of Biotechnology Center for Molecular Neurology, Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium; 4Division of Molecular Design and Synthesis, Department of Chemistry and 6Laboratory of Nephrology, Department of Immunology and Microbiology, Catholic University of Leuven, Leuven, Belgium; and 5Division of Internal Medicine, Nephrology, University Hospitals Leuven, Leuven, Belgium ABSTRACT Background Protein-bound uremic toxins indoxyl sulfate (IS) and p-cresyl sulfate (PCS) have been associ- ated with cardiovascular morbidity and mortality in patients with CKD. However, direct evidence for a role of these toxins in CKD-related vascular calcification has not been reported. Methods To study early and late vascular alterations by toxin exposure, we exposed CKD rats to vehicle, IS (150 mg/kg per day), or PCS (150 mg/kg per day) for either 4 days (short-term exposure) or 7 weeks (long-term exposure). We also performed unbiased proteomic analyses of arterial samples coupled to functional bioinformatic annotation analyses to investigate molecular signaling events associated with toxin-mediated arterial calcification. Results Long-term exposure to either toxin at serum levels similar to those experienced by patients with CKD significantly increased calcification in the aorta and peripheral arteries. -

Anti-ABI1 Monoclonal Antibody, Clone 5F3 (DCABH-351) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-ABI1 monoclonal antibody, clone 5F3 (DCABH-351) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Product Overview Mouse monoclonal to SSH3BP1 Antigen Description May act in negative regulation of cell growth and transformation by interacting with nonreceptor tyrosine kinases ABL1 and/or ABL2. May play a role in regulation of EGF-induced Erk pathway activation. Involved in cytoskeletal reorganization and EGFR signaling. Together with EPS8 participates in transduction of signals from Ras to Rac. In vitro, a trimeric complex of ABI1, EPS8 and SOS1 exhibits Rac specific guanine nucleotide exchange factor (GEF) activity and ABI1 seems to act as an adapter in the complex. Regulates ABL1/c-Abl-mediated phosphorylation of ENAH. Recruits WASF1 to lamellipodia and there seems to regulate WASF1 protein level. Immunogen Recombinant full length protein (Human). Isotype IgG2b Source/Host Mouse Species Reactivity Mouse, Rat, Human Clone 5F3 Purity Tissue culture supernatant Purification Ammonium sulfate precipitated then dialysed in PBS. Conjugate Unconjugated Applications WB, ICC/IF, IHC-Fr, IHC-P, IP Positive Control Prostate Format Liquid Size 100 μl Buffer Preservative: 0.1% Sodium Azide; Constituents: PBS, Tissue Culture Supernatant Preservative 0.1% Sodium Azide Storage store at -20°C or -80°C. Avoid repeated freeze / thaw cycles. Ship Shipped at 4°C. 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics -

UC San Diego Electronic Theses and Dissertations
UC San Diego UC San Diego Electronic Theses and Dissertations Title Cardiac Stretch-Induced Transcriptomic Changes are Axis-Dependent Permalink https://escholarship.org/uc/item/7m04f0b0 Author Buchholz, Kyle Stephen Publication Date 2016 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, SAN DIEGO Cardiac Stretch-Induced Transcriptomic Changes are Axis-Dependent A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Bioengineering by Kyle Stephen Buchholz Committee in Charge: Professor Jeffrey Omens, Chair Professor Andrew McCulloch, Co-Chair Professor Ju Chen Professor Karen Christman Professor Robert Ross Professor Alexander Zambon 2016 Copyright Kyle Stephen Buchholz, 2016 All rights reserved Signature Page The Dissertation of Kyle Stephen Buchholz is approved and it is acceptable in quality and form for publication on microfilm and electronically: Co-Chair Chair University of California, San Diego 2016 iii Dedication To my beautiful wife, Rhia. iv Table of Contents Signature Page ................................................................................................................... iii Dedication .......................................................................................................................... iv Table of Contents ................................................................................................................ v List of Figures ...................................................................................................................