Differential Invasion Success of Salmonids in Southern Chile: Patterns and Hypotheses
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Bristol Bay, Alaska
EPA 910-R-14-001C | January 2014 An Assessment of Potential Mining Impacts on Salmon Ecosystems of Bristol Bay, Alaska Volume 3 – Appendices E-J Region 10, Seattle, WA www.epa.gov/bristolbay EPA 910-R-14-001C January 2014 AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J U.S. Environmental Protection Agency Region 10 Seattle, WA CONTENTS VOLUME 1 An Assessment of Potential Mining Impacts on Salmon Ecosystems of Bristol Bay, Alaska VOLUME 2 APPENDIX A: Fishery Resources of the Bristol Bay Region APPENDIX B: Non-Salmon Freshwater Fishes of the Nushagak and Kvichak River Drainages APPENDIX C: Wildlife Resources of the Nushagak and Kvichak River Watersheds, Alaska APPENDIX D: Traditional Ecological Knowledge and Characterization of the Indigenous Cultures of the Nushagak and Kvichak Watersheds, Alaska VOLUME 3 APPENDIX E: Bristol Bay Wild Salmon Ecosystem: Baseline Levels of Economic Activity and Values APPENDIX F: Biological Characterization: Bristol Bay Marine Estuarine Processes, Fish, and Marine Mammal Assemblages APPENDIX G: Foreseeable Environmental Impact of Potential Road and Pipeline Development on Water Quality and Freshwater Fishery Resources of Bristol Bay, Alaska APPENDIX H: Geologic and Environmental Characteristics of Porphyry Copper Deposits with Emphasis on Potential Future Development in the Bristol Bay Watershed, Alaska APPENDIX I: Conventional Water Quality Mitigation Practices for Mine Design, Construction, Operation, and Closure APPENDIX J: Compensatory Mitigation and Large-Scale Hardrock Mining in the Bristol Bay Watershed AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J Appendix E: Bristol Bay Wild Salmon Ecosystem: Baseline Levels of Economic Activity and Values Bristol Bay Wild Salmon Ecosystem Baseline Levels of Economic Activity and Values John Duffield Chris Neher David Patterson Bioeconomics, Inc. -

Do Some Atlantic Bluefin Tuna Skip Spawning?
SCRS/2006/088 Col. Vol. Sci. Pap. ICCAT, 60(4): 1141-1153 (2007) DO SOME ATLANTIC BLUEFIN TUNA SKIP SPAWNING? David H. Secor1 SUMMARY During the spawning season for Atlantic bluefin tuna, some adults occur outside known spawning centers, suggesting either unknown spawning regions, or fundamental errors in our current understanding of bluefin tuna reproductive schedules. Based upon recent scientific perspectives, skipped spawning (delayed maturation and non-annual spawning) is possibly prevalent in moderately long-lived marine species like bluefin tuna. In principle, skipped spawning represents a trade-off between current and future reproduction. By foregoing reproduction, an individual can incur survival and growth benefits that accrue in deferred reproduction. Across a range of species, skipped reproduction was positively correlated with longevity, but for non-sturgeon species, adults spawned at intervals at least once every two years. A range of types of skipped spawning (constant, younger, older, event skipping; and delays in first maturation) was modeled for the western Atlantic bluefin tuna population to test for their effects on the egg-production-per-recruit biological reference point (stipulated at 20% and 40%). With the exception of extreme delays in maturation, skipped spawning had relatively small effect in depressing fishing mortality (F) threshold values. This was particularly true in comparison to scenarios of a juvenile fishery (ages 4-7), which substantially depressed threshold F values. Indeed, recent F estimates for 1990-2002 western Atlantic bluefin tuna stock assessments were in excess of threshold F values when juvenile size classes were exploited. If western bluefin tuna are currently maturing at an older age than is currently assessed (i.e., 10 v. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

Download Thesis
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ Fast Horses The Racehorse in Health, Disease and Afterlife, 1800 - 1920 Harper, Esther Fiona Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 10. Oct. 2021 Fast Horses: The Racehorse in Health, Disease and Afterlife, 1800 – 1920 Esther Harper Ph.D. History King’s College London April 2018 1 2 Abstract Sports historians have identified the 19th century as a period of significant change in the sport of horseracing, during which it evolved from a sporting pastime of the landed gentry into an industry, and came under increased regulatory control from the Jockey Club. -

Marine Fish Culture
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: © 1998 Kluwer. This manuscript is an author version with the final publication available and may be cited as: Tucker, J. W., Jr. (1998). Marine fish culture. Boston: Kluwer Academic Publishers. MARINE FISH CULTURE by John W. Tucker, Jr., Ph.D. Harbor Branch Oceanographic Institution and Florida Institute of Technology, Melbourne KLUWER ACADEMIC PUBLISHERS Boston I Dordrecht I London Distributors for North, Central and South America: Kluwer Academic Publishers I 01 Philip Drive Assinippi Park Norwell, Massachusetts 02061 USA Telephone (781) 871-6600 Fax (781) 871-6528 E-Mail <[email protected]> Distributors for all other countries: Kluwer Academic Publishers Group Distribution Centre Post Office Box 322 3300 AH Dordrecht, THE NETHERLANDS Telephone 31 78 6392 392 Fax 31 78 6546 474 E-Mail <[email protected]> '' Electronic Services <http://www.wkap.nl> Library of Congress Cataloging-in-Publication Data Tucker, John W., 1948- Marine fish culture I by John W. Tucker, Jr. p. em. Includes bibliographical references (p. ) and index. ISBN 0-412-07151-7 (alk. paper) 1. Marine fishes. 2. Fish-culture. I. Title. SH163.T835 1998 639.3'2--dc21 98-42062 CIP Copyright © 1998 by Kluwer Academic Publishers All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical, photo copying, recording, or otherwise, without the prior written permission of the publisher, Kluwer Academic Publishers, I 0 I Philip Drive, Assinippi Park, Norwell, Massachusetts 02061 Printed on acid-free paper. -

A Preliminary Study on the Stomach Content of Southern Bluefin Tuna Thunnus Maccoyii Caught by Taiwanese Longliner in the Central Indian Ocean
CCSBT-ESC/0509/35 A preliminary study on the stomach content of southern bluefin tuna Thunnus maccoyii caught by Taiwanese longliner in the central Indian Ocean Kwang-Ming Liu1, Wei-Ke Chen2, Shoou-Jeng Joung2, and Sui-Kai Chang3 1. Institute of Marine Resource Management, National Taiwan Ocean University, Keelung, Taiwan. 2. Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan. 3. Fisheries Agency, Council of Agriculture, Taipei, Taiwan. Abstract The stomach contents of 63 southern bluefin tuna captured by Taiwanese longliners in central Indian Ocean in August 2004 were examined. The size of tunas ranged from 84-187 cm FL (12-115 kg GG). The length and weight frequency distributions indicated that most specimens were in the range of 100-130 cm FL with a body weight between 10 and 30 kg for both sexes. The sexes- combined relationship between dressed weight and fork length can be described by W = 6.975× 10-6× FL3.1765 (n=56, r2=0.967, p < 0.05). The subjective index of fullness of specimens was estimated as: 1 = empty (38.6%), 2 = <half full (47.37%), 3 = half full (3.51%), 4 = >half full (5.26%), and 5 = full (5.26%). For the stomachs with prey items, almost all the preys are pisces and the proportion of each prey groups are fishes (95.6%), cephalopods (2.05%), and crustaceans (0.02%). In total, 6 prey taxa were identified – 4 species of fish, 1 unidentified pisces, 1 unidentified crustacean, and 1 unidentified squid. The 4 fish species fall in the family of Carangidae, Clupeidae, Emmelichthyidae, and Hemiramphidae. -

Dominance and Predator Avoidance in Domesticated and Wild Masu Salmon Oncorhynchus Masou
Blackwell Science, LtdOxford, UK FISFisheries Science0919-92682003 Blackwell Science Asia Pty Ltd 691February 2003 591 Behavior of domesticated salmon T Yamamoto and UG Reinhardt 10.1046/j.0919-9268.2002.00591.x Original Article8894BEES SGML FISHERIES SCIENCE 2003; 69: 88–94 Dominance and predator avoidance in domesticated and wild masu salmon Oncorhynchus masou Toshiaki YAMAMOTO* AND Ulrich G REINHARDTa Laboratory of Conservation Biology, Field Science Center for Northern Biosphere, Hokkaido University, Sapporo, Hokkaido 060-0809, Japan ABSTRACT: Dominance, aggression and predator avoidance were compared among farmed, sea- ranched and wild juvenile masu salmon Oncorhynchus masou in laboratory experiments. Domesti- cated fish (farmed and sea-ranched), which had been exposed to artificial selection, were not dominant against wild fish in pairwise contests, nor did they show greater aggressiveness. Farmed fish did show greater feeding than wild fish. Under chemically simulated predation risk, farmed fish were more willing to leave cover and feed than wild fish, indicating reduced predator avoidance in the farmed fish. Our results indicate that selection for fast growth (domestication) in masu salmon favors fish that respond to food quickly and ignore predation risk. KEY WORDS: aggressive behavior, hatchery, masu salmon, predator avoidance. INTRODUCTION impacts the growth of individuals.9 In other salmo- nids, aggressiveness of domesticated juveniles has Numerous studies on salmonids have investigated been shown to increase.10 Therefore, introduced the differences in morphology, genetics and masu juveniles may influence wild populations behavior between domesticated and wild fish of directly through aggressive contests for territories. the same species.1–4 It has become clear that the As masu salmon show strong local adaptations,11,12 introduction of cultured fishes into rivers may there is concern that the introduction of domesti- have negative effects on wild fish populations. -

Lake Tahoe Fish Species
Description: o The Lohonton cutfhroot trout (LCT) is o member of the Solmonidqe {trout ond solmon) fomily, ond is thought to be omong the most endongered western solmonids. o The Lohonton cufihroot wos listed os endongered in 1970 ond reclossified os threotened in 1975. Dork olive bdcks ond reddish to yellow sides frequently chorocterize the LCT found in streoms. Steom dwellers reoch l0 inches in length ond only weigh obout I lb. Their life spon is less thon 5 yeors. ln streoms they ore opportunistic feeders, with diets consisting of drift orgonisms, typicolly terrestriol ond oquotic insects. The sides of loke-dwelling LCT ore often silvery. A brood, pinkish stripe moy be present. Historicolly loke dwellers reoched up to 50 inches in length ond weigh up to 40 pounds. Their life spon is 5-14yeors. ln lokes, smoll Lohontons feed on insects ond zooplonkton while lorger Lohonions feed on other fish. Body spots ore the diognostic chorocter thot distinguishes the Lohonion subspecies from the .l00 Poiute cutthroot. LCT typicolly hove 50 to or more lorge, roundish-block spots thot cover their entire bodies ond their bodies ore typicolly elongoted. o Like other cufihroot trout, they hove bosibronchiol teeth (on the bose of tongue), ond red sloshes under their iow (hence the nome "cutthroot"). o Femole sexuol moturity is reoch between oges of 3 ond 4, while moles moture ot 2 or 3 yeors of oge. o Generolly, they occur in cool flowing woier with ovoiloble cover of well-vegetoted ond stoble streom bonks, in oreos where there ore streom velocity breoks, ond in relotively silt free, rocky riffle-run oreos. -
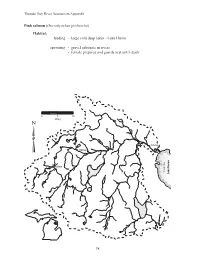
Lake Huron Spawning
Thunder Bay River Assessment Appendix Pink salmon (Oncorhynchus gorbuscha) Habitat: feeding - large cold deep lakes - Lake Huron spawning - gravel substrate in rivers - female prepares and guards nest until death 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 98 Thunder Bay River Assessment Appendix Coho salmon (Oncorhynchus kisutch) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cold streams, later into pools spawning - cold streams and rivers - swifter water of shallow gravelly substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 99 Thunder Bay River Assessment Appendix Rainbow trout (Oncorhynchus mykiss) Habitat: feeding - cold clear water of rivers and Lake Huron - moderate current spawning - gravelly riffles above a pool - smaller tributaries 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 100 Thunder Bay River Assessment Appendix Chinook salmon (Oncorhynchus tshawyscha) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cool streams, later into pools spawning - gravelly substrate in cool streams 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 101 Thunder Bay River Assessment Appendix Round whitefish (Prosopium cylindraceum) Habitat: feeding - lakes, rivers, and streams spawning - shallows of lakes and rivers - gravel or rock substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 102 Thunder Bay River Assessment Appendix Atlantic salmon (Salmo salar) Habitat: feeding - young: gravel substrate streams - adults: Lake Huron -

Salmon Fact Sheet
THE WILD SALMON SEAFOOD MARKET’S GUIDE TO W I L D P A C I F I C S A L M O N Salmon Pacific Salmon occur from northern California along the Pacific Coast throughout the Pacific Ocean, Bering Sea and Arctic Ocean waters adjacent to Alaska. Salmon are anadromous, that is, they spawn in fresh water and the young migrate to the sea where they mature. The mature Salmon returns to the stream of their birth to spawn. Nutrition Few single foods bring as many valuable contributions to the table as Salmon. An excellent source of high-quality protein, containing all the essential amino acids. The fats in Salmon are predominately unsaturated. These fats are evidenced to reduce the risk of heart disease. Availability Although each species has a particular season, small fisheries of wild salmon occur periodically, making fresh salmon (often hard to find and expensive) available throughout the year. Your best values will come during peak salmon season, May through September. Frozen salmon (often frozen at sea) is available during the off season. Also known as Chinook Salmon. Also known as Silver Salmon. Highly desired for King The largest of the species and the most Coho both table use and smoking. Coho salmon offers prominent of the salmon known for its high oil firm meat with excellent flavor slightly milder than content and distinctive, rich flavor. King and Sockeye. Average size from 5 to 40 lbs. Average size from 4 - 9 lbs. Available May - September Available June - September Copper River & Yukon River King Also known as Chum Salmon. -

The 2010-2015 Mega Drought in Central Chile: Impacts on Regional Hydroclimate and Vegetation
Hydrol. Earth Syst. Sci. Discuss., doi:10.5194/hess-2017-191, 2017 Manuscript under review for journal Hydrol. Earth Syst. Sci. Discussion started: 26 April 2017 c Author(s) 2017. CC-BY 3.0 License. The 2010-2015 mega drought in Central Chile: Impacts on regional hydroclimate and vegetation René Garreaud1,2,*, Camila Alvarez-Garreton3,2, Jonathan Barichivich3,2, Juan Pablo Boisier1,2, Duncan 3,2 4,2 3 5 5 Christie , Mauricio Galleguillos , Carlos LeQuesne , James McPhee , Mauricio Zambrano- Bigiarini6,2 1Department of Geophysics, Universidad de Chile, Santiago-Chile 2Center for Climate and Resilience Research (CR2), Santiago-Chile 10 3Laboratorio de Dendrocronología y Cambio Global, Instituto de Conservación Biodiversidad y Territorio, Universidad Austral de Chile, Valdivia-Chile. 4Faculty of Agronomic Sciences, Universidad de Chile, Santiago-Chile 5Department of Civil Engineering, Universidad de Chile, Santiago-Chile 6Department of Civil Engineering, Faculty of Engineering and Sciences, Universidad de La Frontera, Temuco-Chile 15 Correspondence to: René. D. Garreaud ([email protected]) 1 Hydrol. Earth Syst. Sci. Discuss., doi:10.5194/hess-2017-191, 2017 Manuscript under review for journal Hydrol. Earth Syst. Sci. Discussion started: 26 April 2017 c Author(s) 2017. CC-BY 3.0 License. Abstract. Since 2010 an uninterrupted sequence of dry years, with annual rainfall deficits ranging from 25 to 45%, has prevailed in Central Chile (western South America, 30-38°S). Although intense 1- or 2-year droughts are recurrent in this Mediterranean-like region, the ongoing event stands out because of its longevity and large extent. The extraordinary character of the so-called Central Chile mega drought (MD) was established against century long historical records and a 5 millennial tree-ring reconstruction of regional precipitation. -

Imagine the Silver Beauty and the Fighting Spirit of Atlantic Salmon; The
Sakhalin Silver Text and Photos: Clemens Ratschan Imagine the silver beauty and the fighting spirit of Atlantic salmon; the complex, unpredictable life- history of sea trout and combine with the ferocious take and body mass of a predatory taimen. This will give you a glimpse of what fishing for Sakhalin taimen, the silver of the Russian Far East, is about. AM PLEASED TO introduce Siberian taimen, Hucho taimen. No this fish to the readers of wonder, scientists also erroneously Chasing Silver, because in related this far-eastern species to many respects it forms a the large-sized, non-anadromous missing link between the predators of the genus Hucho, which Ifishery for anadromous salmon and is a branch of the salmonoid tree for huchen, a big predatory non- that occurs exclusively in Eurasia. anadromous salmonoid in my home In Central Europe, Hucho hucho is country of Austria (‘Danube salmon’ restricted to the Danube System, in English. See article “Taimen” by where self-sustaining stocks are Wolfgang Hauer, issue 3/2010). presently only found in a handful of Sakhalin taimen is one of the rivers in Germany, Austria, Slovakia least-known salmonid species among and former Yugoslavia. Huchen is non-Russian fishermen; even many very closely related to the already- Russians tend to confuse it with the mentioned Siberian taimen. The latter | 62 | Chasing Silver Fly Fishing Magazine April’s Fav Five www.chasingsilvermagazine.com | 63 | Sakhalin Silver inhabits a distant, vast range from a habits. But one ecological feature expeditions to Japan. Later, the fish few places in European Russia to the is unique – all members of the true was assigned to the genus Parahucho, Lena and Amur rivers in the very far huchen live exclusively in fresh water, with regard to some obvious east of northern Asia.