Notes on Pyrenomycetous Fungi in the Mountain Lake Area of Southwestern Virginia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Phylogeny of Rosellinia Capetribulensis Sp. Nov. and Its Allies (Xylariaceae)
Mycologia, 97(5), 2005, pp. 1102–1110. # 2005 by The Mycological Society of America, Lawrence, KS 66044-8897 Phylogeny of Rosellinia capetribulensis sp. nov. and its allies (Xylariaceae) J. Bahl1 research of the fungi occurring on palms has shown R. Jeewon this particular substrate to be a source of fungal K.D. Hyde diversity (Fro¨hlich and Hyde 2000, Taylor and Hyde Centre for Research in Fungal Diversity, Department of 2003). In continuing studies, we discovered saprobic Ecology & Biodiversity, The University of Hong Kong, fungi on fronds of various palm species (i.e., Pokfulam Road, Hong Kong S.A.R., P.R. China Archontopheonix, Calamus, Livistona) in Northern Queensland and revealed a number of unique fungi. We describe a new species in the genus Rosellinia Abstract: A new Rosellinia species, R. capetribulensis from Calamus sp. isolated from Calamus sp. in Australia is described. R. Most work on Rosellinia has focused on species capetribulensis is characterized by perithecia im- from different geographical regions. Petrini (1992, mersed within a carbonaceous stroma surrounded 2003) compared Rosellinia species from temperate by subiculum-like hyphae, asci with large, barrel- zones and New Zealand. Rogers et al (1987) noted the shaped amyloid apical apparatus and large dark rarity of Rosellinia species in tropical rain forests of brown spores. Morphologically, R. capetribulensis North Sulawesi, Indonesia. In studies of fungi from appears to be similar to R. bunodes, R. markhamiae palm hosts, Smith and Hyde (2001) indexed twelve and R. megalospora. To gain further insights into the Rosellinia species from tropical palm hosts. Rosellinia phylogeny of this new taxon we analyzed the ITS-5.8S species are not frequently isolated when compared to rDNA using maximum parsimony and likelihood other xylariacieous fungi recorded from palm leaf methods. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -

Volatile Constituents of Endophytic Fungi Isolated from Aquilaria Sinensis with Descriptions of Two New Species of Nemania
life Article Volatile Constituents of Endophytic Fungi Isolated from Aquilaria sinensis with Descriptions of Two New Species of Nemania Saowaluck Tibpromma 1,2,3,†, Lu Zhang 4,†, Samantha C. Karunarathna 1,2,3, Tian-Ye Du 1,2,3, Chayanard Phukhamsakda 5,6 , Munikishore Rachakunta 7 , Nakarin Suwannarach 8,9 , Jianchu Xu 1,2,3,*, Peter E. Mortimer 1,2,3,* and Yue-Hu Wang 4,* 1 CAS Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; [email protected] (S.T.); [email protected] (S.C.K.); [email protected] (T.-Y.D.) 2 World Agroforestry Centre, East and Central Asia, Kunming 650201, China 3 Centre for Mountain Futures, Kunming Institute of Botany, Kunming 650201, China 4 Yunnan Key Laboratory for Fungal Diversity and Green Development, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; [email protected] 5 Institute of Plant Protection, College of Agriculture, Jilin Agricultural University, Changchun 130118, China; [email protected] 6 Engineering Research Center of Chinese Ministry of Education for Edible and Medicinal Fungi, Jilin Agricultural University, Changchun 130118, China 7 State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; [email protected] Citation: Tibpromma, S.; Zhang, L.; 8 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand; Karunarathna, S.C.; Du, T.-Y.; [email protected] Phukhamsakda, C.; Rachakunta, M.; 9 Research Center of Microbial Diversity and Sustainable Utilization, Faculty of Science, Chiang Mai University, Suwannarach, N.; Xu, J.; Mortimer, Chiang Mai 50200, Thailand P.E.; Wang, Y.-H. -

Lichens and Associated Fungi from Glacier Bay National Park, Alaska
The Lichenologist (2020), 52,61–181 doi:10.1017/S0024282920000079 Standard Paper Lichens and associated fungi from Glacier Bay National Park, Alaska Toby Spribille1,2,3 , Alan M. Fryday4 , Sergio Pérez-Ortega5 , Måns Svensson6, Tor Tønsberg7, Stefan Ekman6 , Håkon Holien8,9, Philipp Resl10 , Kevin Schneider11, Edith Stabentheiner2, Holger Thüs12,13 , Jan Vondrák14,15 and Lewis Sharman16 1Department of Biological Sciences, CW405, University of Alberta, Edmonton, Alberta T6G 2R3, Canada; 2Department of Plant Sciences, Institute of Biology, University of Graz, NAWI Graz, Holteigasse 6, 8010 Graz, Austria; 3Division of Biological Sciences, University of Montana, 32 Campus Drive, Missoula, Montana 59812, USA; 4Herbarium, Department of Plant Biology, Michigan State University, East Lansing, Michigan 48824, USA; 5Real Jardín Botánico (CSIC), Departamento de Micología, Calle Claudio Moyano 1, E-28014 Madrid, Spain; 6Museum of Evolution, Uppsala University, Norbyvägen 16, SE-75236 Uppsala, Sweden; 7Department of Natural History, University Museum of Bergen Allégt. 41, P.O. Box 7800, N-5020 Bergen, Norway; 8Faculty of Bioscience and Aquaculture, Nord University, Box 2501, NO-7729 Steinkjer, Norway; 9NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway; 10Faculty of Biology, Department I, Systematic Botany and Mycology, University of Munich (LMU), Menzinger Straße 67, 80638 München, Germany; 11Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; 12Botany Department, State Museum of Natural History Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany; 13Natural History Museum, Cromwell Road, London SW7 5BD, UK; 14Institute of Botany of the Czech Academy of Sciences, Zámek 1, 252 43 Průhonice, Czech Republic; 15Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic and 16Glacier Bay National Park & Preserve, P.O. -

<I>Abieticola Koreana</I>
MYCOTAXON ISSN (print) 0093-4666 (online) 2154-8889 © 2016. Mycotaxon, Ltd. October–December 2016—Volume 131, pp. 749–764 http://dx.doi.org/10.5248/131.749 Abieticola koreana gen. et sp. nov., a griseofulvin-producing endophytic xylariaceous ascomycete from Korea Hyang Burm Lee1*, Hye Yeon Mun1,2, Thi Thuong Thuong Nguyen1, Jin-Cheol Kim1 & Jeffrey K. Stone3 1College of Agriculture & Life Sciences, Chonnam National University, Gwangju 61186, Republic of Korea 2Fungal Resources Research Division, Nakdonggang National Institute of Biological Resources, Sangju 37242, Republic of Korea 3Department of Botany & Plant Pathology, Cordley Hall 2082, Oregon State University, Corvallis, OR 97331-2902, USA *Correspondence to: [email protected] Abstract—A new genus and species. Abieticola koreana, is described. This xylariaceous fungus was isolated from the inner bark of a Manchurian fir (Abies holophylla) in Korea. Phylogenetic analyses based on the sequences of four gene regions—ITS1-5.8S-ITS2, 28S, β-tubulin, and rpb2—were used to confirm this new genus and species. Key words—Ascomycota, anamorph, multigene, Xylarioideae, Xylariaceae Introduction The Xylariaceae is a large family of ascomycetes comprising approximately 85 genera and at least 1,340 species that are distributed worldwide and exhibit exceptional diversity in the tropics (Whalley 1996, Velmurugan et al. 2013, Stadler et al. 2014). It has been estimated that the Xylariaceae contains 10,000 undescribed species (Stadler 2011, Richardson et al. 2014). Recent phylogenetic studies (Tang et al. 2009, Daranagama et al. 2015) using combined ITS, LSU, rpb2, and β-tubulin sequences, suggested that Xylariaceae has two major lineages, Xylarioideae and Hypoxyloideae. To date, 11 genera of Xylariaceae have been shown to occur as endophytes of various plants (Pažoutová et al. -

Resurrection and Emendation of the Hypoxylaceae, Recognised from a Multigene Phylogeny of the Xylariales
Mycol Progress DOI 10.1007/s11557-017-1311-3 ORIGINAL ARTICLE Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales Lucile Wendt1,2 & Esteban Benjamin Sir3 & Eric Kuhnert1,2 & Simone Heitkämper1,2 & Christopher Lambert1,2 & Adriana I. Hladki3 & Andrea I. Romero4,5 & J. Jennifer Luangsa-ard6 & Prasert Srikitikulchai6 & Derek Peršoh7 & Marc Stadler1,2 Received: 21 February 2017 /Revised: 12 April 2017 /Accepted: 19 April 2017 # The Author(s) 2017. This article is an open access publication Abstract A multigene phylogeny was constructed, including polymerase II (RPB2), and beta-tubulin (TUB2). Specimens a significant number of representative species of the main were selected based on more than a decade of intensive mor- lineages in the Xylariaceae and four DNA loci the internal phological and chemotaxonomic work, and cautious taxon transcribed spacer region (ITS), the large subunit (LSU) of sampling was performed to cover the major lineages of the the nuclear rDNA, the second largest subunit of the RNA Xylariaceae; however, with emphasis on hypoxyloid species. The comprehensive phylogenetic analysis revealed a clear-cut This article is part of the “Special Issue on ascomycete systematics in segregation of the Xylariaceae into several major clades, honor of Richard P. Korf who died in August 2016”. which was well in accordance with previously established morphological and chemotaxonomic concepts. One of these The present paper is dedicated to Prof. Jack D. Rogers, on the occasion of his fortcoming 80th birthday. clades contained Annulohypoxylon, Hypoxylon, Daldinia,and other related genera that have stromatal pigments and a Section Editor: Teresa Iturriaga and Marc Stadler nodulisporium-like anamorph. -

Identification of Rosellinia Species As Producers Of
available online at www.studiesinmycology.org STUDIES IN MYCOLOGY 96: 1–16 (2020). Identification of Rosellinia species as producers of cyclodepsipeptide PF1022 A and resurrection of the genus Dematophora as inferred from polythetic taxonomy K. Wittstein1,2, A. Cordsmeier1,3, C. Lambert1,2, L. Wendt1,2, E.B. Sir4, J. Weber1,2, N. Wurzler1,2, L.E. Petrini5, and M. Stadler1,2* 1Helmholtz-Zentrum für Infektionsforschung GmbH, Department Microbial Drugs, Inhoffenstrasse 7, Braunschweig, 38124, Germany; 2German Centre for Infection Research (DZIF), Partner site Hannover-Braunschweig, Braunschweig, 38124, Germany; 3University Hospital Erlangen, Institute of Microbiology - Clinical Microbiology, Immunology and Hygiene, Wasserturmstraße 3/5, Erlangen, 91054, Germany; 4Instituto de Bioprospeccion y Fisiología Vegetal-INBIOFIV (CONICET- UNT), San Lorenzo 1469, San Miguel de Tucuman, Tucuman, 4000, Argentina; 5Via al Perato 15c, Breganzona, CH-6932, Switzerland *Correspondence: M. Stadler, [email protected] Abstract: Rosellinia (Xylariaceae) is a large, cosmopolitan genus comprising over 130 species that have been defined based mainly on the morphology of their sexual morphs. The genus comprises both lignicolous and saprotrophic species that are frequently isolated as endophytes from healthy host plants, and important plant pathogens. In order to evaluate the utility of molecular phylogeny and secondary metabolite profiling to achieve a better basis for their classification, a set of strains was selected for a multi-locus phylogeny inferred from a combination of the sequences of the internal transcribed spacer region (ITS), the large subunit (LSU) of the nuclear rDNA, beta-tubulin (TUB2) and the second largest subunit of the RNA polymerase II (RPB2). Concurrently, various strains were surveyed for production of secondary metabolites. -

UC Riverside UC Riverside Previously Published Works
UC Riverside UC Riverside Previously Published Works Title Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Permalink https://escholarship.org/uc/item/3fm155t1 Authors U'Ren, Jana M Miadlikowska, Jolanta Zimmerman, Naupaka B et al. Publication Date 2016-05-01 DOI 10.1016/j.ympev.2016.02.010 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California *Graphical Abstract (for review) ! *Highlights (for review) • Endophytes illuminate Xylariaceae circumscription and phylogenetic structure. • Endophytes occur in lineages previously not known for endophytism. • Boreal and temperate lichens and non-flowering plants commonly host Xylariaceae. • Many have endophytic and saprotrophic life stages and are widespread generalists. *Manuscript Click here to view linked References 1 Contributions of North American endophytes to the phylogeny, 2 ecology, and taxonomy of Xylariaceae (Sordariomycetes, 3 Ascomycota) 4 5 6 Jana M. U’Ren a,* Jolanta Miadlikowska b, Naupaka B. Zimmerman a, François Lutzoni b, Jason 7 E. Stajichc, and A. Elizabeth Arnold a,d 8 9 10 a University of Arizona, School of Plant Sciences, 1140 E. South Campus Dr., Forbes 303, 11 Tucson, AZ 85721, USA 12 b Duke University, Department of Biology, Durham, NC 27708-0338, USA 13 c University of California-Riverside, Department of Plant Pathology and Microbiology and Institute 14 for Integrated Genome Biology, 900 University Ave., Riverside, CA 92521, USA 15 d University of Arizona, Department of Ecology and Evolutionary Biology, 1041 E. Lowell St., 16 BioSciences West 310, Tucson, AZ 85721, USA 17 18 19 20 21 22 23 24 * Corresponding author: University of Arizona, School of Plant Sciences, 1140 E. -

Molecular Systematics of the Sordariales: the Order and the Family Lasiosphaeriaceae Redefined
Mycologia, 96(2), 2004, pp. 368±387. q 2004 by The Mycological Society of America, Lawrence, KS 66044-8897 Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae rede®ned Sabine M. Huhndorf1 other families outside the Sordariales and 22 addi- Botany Department, The Field Museum, 1400 S. Lake tional genera with differing morphologies subse- Shore Drive, Chicago, Illinois 60605-2496 quently are transferred out of the order. Two new Andrew N. Miller orders, Coniochaetales and Chaetosphaeriales, are recognized for the families Coniochaetaceae and Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 Chaetosphaeriaceae respectively. The Boliniaceae is University of Illinois at Chicago, Department of accepted in the Boliniales, and the Nitschkiaceae is Biological Sciences, Chicago, Illinois 60607-7060 accepted in the Coronophorales. Annulatascaceae and Cephalothecaceae are placed in Sordariomyce- Fernando A. FernaÂndez tidae inc. sed., and Batistiaceae is placed in the Euas- Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 comycetes inc. sed. Key words: Annulatascaceae, Batistiaceae, Bolini- aceae, Catabotrydaceae, Cephalothecaceae, Ceratos- Abstract: The Sordariales is a taxonomically diverse tomataceae, Chaetomiaceae, Coniochaetaceae, Hel- group that has contained from seven to 14 families minthosphaeriaceae, LSU nrDNA, Nitschkiaceae, in recent years. The largest family is the Lasiosphaer- Sordariaceae iaceae, which has contained between 33 and 53 gen- era, depending on the chosen classi®cation. To de- termine the af®nities and taxonomic placement of INTRODUCTION the Lasiosphaeriaceae and other families in the Sor- The Sordariales is one of the most taxonomically di- dariales, taxa representing every family in the Sor- verse groups within the Class Sordariomycetes (Phy- dariales and most of the genera in the Lasiosphaeri- lum Ascomycota, Subphylum Pezizomycotina, ®de aceae were targeted for phylogenetic analysis using Eriksson et al 2001). -
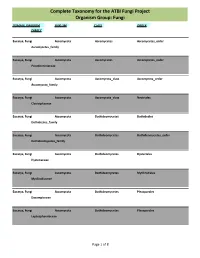
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Ascomycetes_family Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Pseudeurotiaceae Eucarya, Fungi Ascomycota Ascomycota_class Ascomycota_order Ascomycota_family Eucarya, Fungi Ascomycota Ascomycota_class Nectriales Clavicipitaceae Eucarya, Fungi Ascomycota Dothideomycetes Dothideales Dothideales_family Eucarya, Fungi Ascomycota Dothideomycetes Dothideomycetes_order Dothideomycetes_family Eucarya, Fungi Ascomycota Dothideomycetes Hysteriales Hysteriaceae Eucarya, Fungi Ascomycota Dothideomycetes Mytilinidiales Mytilinidiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Dacampiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Leptosphaeriaceae Page 1 of 8 Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Lophiostomataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Massarinaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Melanommataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleomassariaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales_family Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Sporormiaceae Eucarya, Fungi Ascomycota Dothideomycetes -

Diverse Partitiviruses from the Phytopathogenic Fungus, Rosellinia Necatrix
ORIGINAL RESEARCH published: 26 June 2020 doi: 10.3389/fmicb.2020.01064 Diverse Partitiviruses From the Phytopathogenic Fungus, Rosellinia necatrix Paul Telengech 1, Sakae Hisano 1, Cyrus Mugambi 1, Kiwamu Hyodo 1, Juan Manuel Arjona-López 1,2, Carlos José López-Herrera 2, Satoko Kanematsu 3†, Hideki Kondo 1 and Nobuhiro Suzuki 1* 1 Institute of Plant Science and Resources, Okayama University, Kurashiki, Japan, 2 Institute for Sustainable Agriculture, Spanish Research Council, Córdoba, Spain, 3 Institute of Fruit Tree Science, National Agriculture and Food Research Organization (NARO), Morioka, Japan Partitiviruses (dsRNA viruses, family Partitiviridae) are ubiquitously detected in plants and fungi. Although previous surveys suggested their omnipresence in the white root rot fungus, Rosellinia necatrix, only a few of them have been molecularly and biologically Edited by: characterized thus far. We report the characterization of a total of 20 partitiviruses from 16 Hiromitsu Moriyama, Tokyo University of Agriculture and R. necatrix strains belonging to 15 new species, for which “Rosellinia necatrix partitivirus Technology, Japan 11–Rosellinia necatrix partitivirus 25” were proposed, and 5 previously reported species. Reviewed by: The newly identified partitiviruses have been taxonomically placed in two genera, Eeva Johanna Vainio, Natural Resources Institute Alphapartitivirus, and Betapartitivirus. Some partitiviruses were transfected into reference Finland, Finland strains of the natural host, R. necatrix, and an experimental host, Cryphonectria K. W. Thilini Chethana, parasitica, using purified virions. A comparative analysis of resultant transfectants Mae Fah Luang University, Thailand revealed interesting differences and similarities between the RNA accumulation and *Correspondence: Nobuhiro Suzuki symptom induction patterns of R. necatrix and C. parasitica. Other interesting findings [email protected] include the identification of a probable reassortment event and a quintuple partitivirus †Present address: infection of a single fungal strain. -

Biogeography and Taxonomy of Pyrenomycetous Fungi 3. the Area Around the Sea of Japan
ISSN (print) 0093-4666 © 2013. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/126.1 Volume 126, pp. 1–14 October–December 2013 Biogeography and taxonomy of pyrenomycetous fungi 3. The area around the Sea of Japan Larissa N. Vasilyeva1*, Hai-xia Ma2,3 & Steven L. Stephenson4 1Institute of Biology & Soil Science, Far East Branch of the Russian Academy of Sciences, Vladivostok 690022, Russia 2Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, China 3Institute of Mycology, Jilin Agricultural University, Changchun 130118, China 4Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA *Correspondence to: [email protected] Abstract — The peculiar species composition of the assemblage of pyrenomycetous fungi known from the area around the Sea of Japan is discussed. Three new species—Annulohypoxylon orientale, Hypoxylon cyanescens, and Nectria araliae—are described and illustrated. Key words — Ascomycota, northeastern Asia, ecological distribution Introduction One of the most interesting regions of northeastern Asia is the area around the Sea of Japan. Many pyrenomycetous fungi are restricted to this region of the world, and a number of economically important pathogenic fungi are known only from northeastern China, Japan, Korea and southeastern Russia. Unfortunately, often many of these fungi have been confused with other species, so phytopathologists cannot recognize them and thus develop the appropriate control measures. At the 2011 Asian Mycological Congress held in Incheon (Korea), Dr. Tsuyoshi Hosoya—during his talk on the reassessment of Hyaloscyphaceae (Helotiales, Leotiomycetes) based on molecular phylogenetic analysis—repeated Dr. Richard Korf’s statement that “for the discosystematist, Japan is a relatively unexplored paradise” and that the region is “rich in apparently endemic forms, an understanding of which is essential for phylogenetic and phytogeographic studies” (Korf 1958: 7).