Stray Studies in the Coronophorales (Pyrenomycetes) 4-8
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lichens and Associated Fungi from Glacier Bay National Park, Alaska
The Lichenologist (2020), 52,61–181 doi:10.1017/S0024282920000079 Standard Paper Lichens and associated fungi from Glacier Bay National Park, Alaska Toby Spribille1,2,3 , Alan M. Fryday4 , Sergio Pérez-Ortega5 , Måns Svensson6, Tor Tønsberg7, Stefan Ekman6 , Håkon Holien8,9, Philipp Resl10 , Kevin Schneider11, Edith Stabentheiner2, Holger Thüs12,13 , Jan Vondrák14,15 and Lewis Sharman16 1Department of Biological Sciences, CW405, University of Alberta, Edmonton, Alberta T6G 2R3, Canada; 2Department of Plant Sciences, Institute of Biology, University of Graz, NAWI Graz, Holteigasse 6, 8010 Graz, Austria; 3Division of Biological Sciences, University of Montana, 32 Campus Drive, Missoula, Montana 59812, USA; 4Herbarium, Department of Plant Biology, Michigan State University, East Lansing, Michigan 48824, USA; 5Real Jardín Botánico (CSIC), Departamento de Micología, Calle Claudio Moyano 1, E-28014 Madrid, Spain; 6Museum of Evolution, Uppsala University, Norbyvägen 16, SE-75236 Uppsala, Sweden; 7Department of Natural History, University Museum of Bergen Allégt. 41, P.O. Box 7800, N-5020 Bergen, Norway; 8Faculty of Bioscience and Aquaculture, Nord University, Box 2501, NO-7729 Steinkjer, Norway; 9NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway; 10Faculty of Biology, Department I, Systematic Botany and Mycology, University of Munich (LMU), Menzinger Straße 67, 80638 München, Germany; 11Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; 12Botany Department, State Museum of Natural History Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany; 13Natural History Museum, Cromwell Road, London SW7 5BD, UK; 14Institute of Botany of the Czech Academy of Sciences, Zámek 1, 252 43 Průhonice, Czech Republic; 15Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic and 16Glacier Bay National Park & Preserve, P.O. -

A Higher-Level Phylogenetic Classification of the Fungi
mycological research 111 (2007) 509–547 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/mycres A higher-level phylogenetic classification of the Fungi David S. HIBBETTa,*, Manfred BINDERa, Joseph F. BISCHOFFb, Meredith BLACKWELLc, Paul F. CANNONd, Ove E. ERIKSSONe, Sabine HUHNDORFf, Timothy JAMESg, Paul M. KIRKd, Robert LU¨ CKINGf, H. THORSTEN LUMBSCHf, Franc¸ois LUTZONIg, P. Brandon MATHENYa, David J. MCLAUGHLINh, Martha J. POWELLi, Scott REDHEAD j, Conrad L. SCHOCHk, Joseph W. SPATAFORAk, Joost A. STALPERSl, Rytas VILGALYSg, M. Catherine AIMEm, Andre´ APTROOTn, Robert BAUERo, Dominik BEGEROWp, Gerald L. BENNYq, Lisa A. CASTLEBURYm, Pedro W. CROUSl, Yu-Cheng DAIr, Walter GAMSl, David M. GEISERs, Gareth W. GRIFFITHt,Ce´cile GUEIDANg, David L. HAWKSWORTHu, Geir HESTMARKv, Kentaro HOSAKAw, Richard A. HUMBERx, Kevin D. HYDEy, Joseph E. IRONSIDEt, Urmas KO˜ LJALGz, Cletus P. KURTZMANaa, Karl-Henrik LARSSONab, Robert LICHTWARDTac, Joyce LONGCOREad, Jolanta MIA˛ DLIKOWSKAg, Andrew MILLERae, Jean-Marc MONCALVOaf, Sharon MOZLEY-STANDRIDGEag, Franz OBERWINKLERo, Erast PARMASTOah, Vale´rie REEBg, Jack D. ROGERSai, Claude ROUXaj, Leif RYVARDENak, Jose´ Paulo SAMPAIOal, Arthur SCHU¨ ßLERam, Junta SUGIYAMAan, R. Greg THORNao, Leif TIBELLap, Wendy A. UNTEREINERaq, Christopher WALKERar, Zheng WANGa, Alex WEIRas, Michael WEISSo, Merlin M. WHITEat, Katarina WINKAe, Yi-Jian YAOau, Ning ZHANGav aBiology Department, Clark University, Worcester, MA 01610, USA bNational Library of Medicine, National Center for Biotechnology Information, -

Molecular Systematics of the Coronophorales and New Species of Bertia, Lasiobertia and Nitschkia
Mycol. Res. 108 (12): 1384–1398 (December 2004). f The British Mycological Society 1384 DOI: 10.1017/S0953756204001273 Printed in the United Kingdom. Molecular systematics of the Coronophorales and new species of Bertia, Lasiobertia and Nitschkia Sabine M. HUHNDORF, Andrew N. MILLER* and Fernando A. FERNA´NDEZ The Field Museum of Natural History, Botany Department, Chicago, Illinois 60605-2496, USA. E-mail : [email protected] Received 16 April 2004; accepted 11 August 2004. The Nitschkiaceae has been placed in the Coronophorales or the Sordariales in recent years. Most recently it was accepted in the Coronophorales and placed in the Hypocreomycetidae based on sequence data from large subunit nrDNA. To confirm and corroborate the taxonomic placement and monophyly of the Coronophorales, additional taxa representing the diversity of the group were targeted for phylogenetic analysis using partial sequences of the large subunit nrDNA (LSU). Based on molecular data, the Coronophorales is found to be monophyletic and its placement in the Hypocreomycetidae is maintained. The order is a coherent group with morphologies that include superficial, often turbinate, often collabent ascomata that may or may not contain a quellkorper and asci that are often stipitate and at times polysporous. Three species with accepted Nitschkia names, together with Fracchiaea broomeiana and Acanthonitschkea argentinensis, comprise the paraphyletic nitschkiaceous complex. Two new families, Chaetosphaerellaceae and Scortechiniaceae fams nov., are described for the clades containing Chaetosphaerella and Crassochaeta and the taxa having a quellkorper (Euacanthe, Neofracchiaea and Scortechinia) respectively. The Bertiaceae is accepted for the clade containing Bertia species. Three new species are described: Bertia tropicalis, Lasiobertia portoricensis, and Nitschkia meniscoidea spp. -

Savoryellales (Hypocreomycetidae, Sordariomycetes): a Novel Lineage
Mycologia, 103(6), 2011, pp. 1351–1371. DOI: 10.3852/11-102 # 2011 by The Mycological Society of America, Lawrence, KS 66044-8897 Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella Nattawut Boonyuen1 Canalisporium) formed a new lineage that has Mycology Laboratory (BMYC), Bioresources Technology invaded both marine and freshwater habitats, indi- Unit (BTU), National Center for Genetic Engineering cating that these genera share a common ancestor and Biotechnology (BIOTEC), 113 Thailand Science and are closely related. Because they show no clear Park, Phaholyothin Road, Khlong 1, Khlong Luang, Pathumthani 12120, Thailand, and Department of relationship with any named order we erect a new Plant Pathology, Faculty of Agriculture, Kasetsart order Savoryellales in the subclass Hypocreomyceti- University, 50 Phaholyothin Road, Chatuchak, dae, Sordariomycetes. The genera Savoryella and Bangkok 10900, Thailand Ascothailandia are monophyletic, while the position Charuwan Chuaseeharonnachai of Ascotaiwania is unresolved. All three genera are Satinee Suetrong phylogenetically related and form a distinct clade Veera Sri-indrasutdhi similar to the unclassified group of marine ascomy- Somsak Sivichai cetes comprising the genera Swampomyces, Torpedos- E.B. Gareth Jones pora and Juncigera (TBM clade: Torpedospora/Bertia/ Mycology Laboratory (BMYC), Bioresources Technology Melanospora) in the Hypocreomycetidae incertae -

Molecular Systematics of the Sordariales: the Order and the Family Lasiosphaeriaceae Redefined
Mycologia, 96(2), 2004, pp. 368±387. q 2004 by The Mycological Society of America, Lawrence, KS 66044-8897 Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae rede®ned Sabine M. Huhndorf1 other families outside the Sordariales and 22 addi- Botany Department, The Field Museum, 1400 S. Lake tional genera with differing morphologies subse- Shore Drive, Chicago, Illinois 60605-2496 quently are transferred out of the order. Two new Andrew N. Miller orders, Coniochaetales and Chaetosphaeriales, are recognized for the families Coniochaetaceae and Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 Chaetosphaeriaceae respectively. The Boliniaceae is University of Illinois at Chicago, Department of accepted in the Boliniales, and the Nitschkiaceae is Biological Sciences, Chicago, Illinois 60607-7060 accepted in the Coronophorales. Annulatascaceae and Cephalothecaceae are placed in Sordariomyce- Fernando A. FernaÂndez tidae inc. sed., and Batistiaceae is placed in the Euas- Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 comycetes inc. sed. Key words: Annulatascaceae, Batistiaceae, Bolini- aceae, Catabotrydaceae, Cephalothecaceae, Ceratos- Abstract: The Sordariales is a taxonomically diverse tomataceae, Chaetomiaceae, Coniochaetaceae, Hel- group that has contained from seven to 14 families minthosphaeriaceae, LSU nrDNA, Nitschkiaceae, in recent years. The largest family is the Lasiosphaer- Sordariaceae iaceae, which has contained between 33 and 53 gen- era, depending on the chosen classi®cation. To de- termine the af®nities and taxonomic placement of INTRODUCTION the Lasiosphaeriaceae and other families in the Sor- The Sordariales is one of the most taxonomically di- dariales, taxa representing every family in the Sor- verse groups within the Class Sordariomycetes (Phy- dariales and most of the genera in the Lasiosphaeri- lum Ascomycota, Subphylum Pezizomycotina, ®de aceae were targeted for phylogenetic analysis using Eriksson et al 2001). -
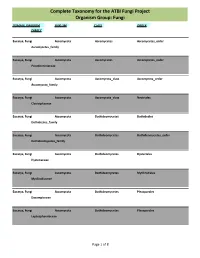
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Ascomycetes_family Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Pseudeurotiaceae Eucarya, Fungi Ascomycota Ascomycota_class Ascomycota_order Ascomycota_family Eucarya, Fungi Ascomycota Ascomycota_class Nectriales Clavicipitaceae Eucarya, Fungi Ascomycota Dothideomycetes Dothideales Dothideales_family Eucarya, Fungi Ascomycota Dothideomycetes Dothideomycetes_order Dothideomycetes_family Eucarya, Fungi Ascomycota Dothideomycetes Hysteriales Hysteriaceae Eucarya, Fungi Ascomycota Dothideomycetes Mytilinidiales Mytilinidiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Dacampiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Leptosphaeriaceae Page 1 of 8 Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Lophiostomataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Massarinaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Melanommataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleomassariaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales_family Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Sporormiaceae Eucarya, Fungi Ascomycota Dothideomycetes -

Multigene Phylogeny of the Coronophorales: Morphology and New Species in the Order
Mycologia, 102(1), 2010, pp. 185–210. DOI: 10.3852/09-043 # 2010 by The Mycological Society of America, Lawrence, KS 66044-8897 Multigene phylogeny of the Coronophorales: morphology and new species in the order George K. Mugambi1 an important character in defining the Scortechinia- Botany Department, Field Museum of Natural History, ceae, while taxa within the group show a mixture of Chicago, Illinois 60605–2496, Department of morphological characteristics of varying phylogenetic Biological Sciences, University of Illinois at Chicago, importance. The presence of smooth versus spinulose 845 W. Taylor Street (MC 066), Chicago, IL 60607, and National Museums of Kenya, Botany Department, subiculum aids in separating Tympanopsis and P.O. Box 45166, 00100, Nairobi, Kenya Scortechinia, and erumpent ascomata distinguish Cryptosphaerella species. Taxa within the Bertiaceae Sabine M. Huhndorf vary along the lines of robust, tuberculate, collapsing Botany Department, Field Museum of Natural History, Chicago, Illinois 60605–2496 ascomata and large, hyaline to pigmented, septate ascospores. Key words: Ascomycota, Coronophorales, LSU Abstract: The phylogenetic relationships within Cor- rDNA, phylogeny, quellko¨rper, rpb2, tef1 onophorales have been debated because of uncer- tainty over the taxonomic usefulness of characteristics INTRODUCTION such as quellko¨rper, number of ascospores per ascus, presence of ascospore appendages, presence of Members of the Coronophorales are common wood- subiculum and ascomatal vestiture. The phylogenetic inhabiting Ascomycete fungi with a worldwide distri- relationships are examined with DNA sequence data bution. Coronophorales is characterized by taxa with from three nuclear genes targeting 69 taxa and 130 mostly superficial ascomata, sometimes with an new sequences representing collections from Africa extensive hyphal subiculum or well developed basal and the Americas. -

Monilochaetes and Allied Genera of the Glomerellales, and a Reconsideration of Families in the Microascales
available online at www.studiesinmycology.org StudieS in Mycology 68: 163–191. 2011. doi:10.3114/sim.2011.68.07 Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales M. Réblová1*, W. Gams2 and K.A. Seifert3 1Department of Taxonomy, Institute of Botany of the Academy of Sciences, CZ – 252 43 Průhonice, Czech Republic; 2Molenweg 15, 3743CK Baarn, The Netherlands; 3Biodiversity (Mycology and Botany), Agriculture and Agri-Food Canada, Ottawa, Ontario, K1A 0C6, Canada *Correspondence: Martina Réblová, [email protected] Abstract: We examined the phylogenetic relationships of two species that mimic Chaetosphaeria in teleomorph and anamorph morphologies, Chaetosphaeria tulasneorum with a Cylindrotrichum anamorph and Australiasca queenslandica with a Dischloridium anamorph. Four data sets were analysed: a) the internal transcribed spacer region including ITS1, 5.8S rDNA and ITS2 (ITS), b) nc28S (ncLSU) rDNA, c) nc18S (ncSSU) rDNA, and d) a combined data set of ncLSU-ncSSU-RPB2 (ribosomal polymerase B2). The traditional placement of Ch. tulasneorum in the Microascales based on ncLSU sequences is unsupported and Australiasca does not belong to the Chaetosphaeriaceae. Both holomorph species are nested within the Glomerellales. A new genus, Reticulascus, is introduced for Ch. tulasneorum with associated Cylindrotrichum anamorph; another species of Reticulascus and its anamorph in Cylindrotrichum are described as new. The taxonomic structure of the Glomerellales is clarified and the name is validly published. As delimited here, it includes three families, the Glomerellaceae and the newly described Australiascaceae and Reticulascaceae. Based on ITS and ncLSU rDNA sequence analyses, we confirm the synonymy of the anamorph generaDischloridium with Monilochaetes. -

An Overview of the Systematics of the Sordariomycetes Based on a Four-Gene Phylogeny
Mycologia, 98(6), 2006, pp. 1076–1087. # 2006 by The Mycological Society of America, Lawrence, KS 66044-8897 An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny Ning Zhang of 16 in the Sordariomycetes was investigated based Department of Plant Pathology, NYSAES, Cornell on four nuclear loci (nSSU and nLSU rDNA, TEF and University, Geneva, New York 14456 RPB2), using three species of the Leotiomycetes as Lisa A. Castlebury outgroups. Three subclasses (i.e. Hypocreomycetidae, Systematic Botany & Mycology Laboratory, USDA-ARS, Sordariomycetidae and Xylariomycetidae) currently Beltsville, Maryland 20705 recognized in the classification are well supported with the placement of the Lulworthiales in either Andrew N. Miller a basal group of the Sordariomycetes or a sister group Center for Biodiversity, Illinois Natural History Survey, of the Hypocreomycetidae. Except for the Micro- Champaign, Illinois 61820 ascales, our results recognize most of the orders as Sabine M. Huhndorf monophyletic groups. Melanospora species form Department of Botany, The Field Museum of Natural a clade outside of the Hypocreales and are recognized History, Chicago, Illinois 60605 as a distinct order in the Hypocreomycetidae. Conrad L. Schoch Glomerellaceae is excluded from the Phyllachorales Department of Botany and Plant Pathology, Oregon and placed in Hypocreomycetidae incertae sedis. In State University, Corvallis, Oregon 97331 the Sordariomycetidae, the Sordariales is a strongly supported clade and occurs within a well supported Keith A. Seifert clade containing the Boliniales and Chaetosphaer- Biodiversity (Mycology and Botany), Agriculture and iales. Aspects of morphology, ecology and evolution Agri-Food Canada, Ottawa, Ontario, K1A 0C6 Canada are discussed. Amy Y. -

Biogeography and Taxonomy of Pyrenomycetous Fungi 3. the Area Around the Sea of Japan
ISSN (print) 0093-4666 © 2013. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/126.1 Volume 126, pp. 1–14 October–December 2013 Biogeography and taxonomy of pyrenomycetous fungi 3. The area around the Sea of Japan Larissa N. Vasilyeva1*, Hai-xia Ma2,3 & Steven L. Stephenson4 1Institute of Biology & Soil Science, Far East Branch of the Russian Academy of Sciences, Vladivostok 690022, Russia 2Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, China 3Institute of Mycology, Jilin Agricultural University, Changchun 130118, China 4Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA *Correspondence to: [email protected] Abstract — The peculiar species composition of the assemblage of pyrenomycetous fungi known from the area around the Sea of Japan is discussed. Three new species—Annulohypoxylon orientale, Hypoxylon cyanescens, and Nectria araliae—are described and illustrated. Key words — Ascomycota, northeastern Asia, ecological distribution Introduction One of the most interesting regions of northeastern Asia is the area around the Sea of Japan. Many pyrenomycetous fungi are restricted to this region of the world, and a number of economically important pathogenic fungi are known only from northeastern China, Japan, Korea and southeastern Russia. Unfortunately, often many of these fungi have been confused with other species, so phytopathologists cannot recognize them and thus develop the appropriate control measures. At the 2011 Asian Mycological Congress held in Incheon (Korea), Dr. Tsuyoshi Hosoya—during his talk on the reassessment of Hyaloscyphaceae (Helotiales, Leotiomycetes) based on molecular phylogenetic analysis—repeated Dr. Richard Korf’s statement that “for the discosystematist, Japan is a relatively unexplored paradise” and that the region is “rich in apparently endemic forms, an understanding of which is essential for phylogenetic and phytogeographic studies” (Korf 1958: 7). -

An Additional Fungal Lineage in the Hypocreomycetidae (Falcocladium Species) and the Taxonomic Re-Evaluation of Chaetosphaeria C
Cryptogamie, Mycologie, 2014, 35 (2): 119-138 © 2014 Adac. Tous droits réservés An additional fungal lineage in the Hypocreomycetidae (Falcocladium species) and the taxonomic re-evaluation of Chaetosphaeria chaetosa and Swampomyces species, based on morphology, ecology and phylogeny E.B. Gareth JONESa*, Satinee SUETRONGb, Wan-Hsuan CHENGc, Nattawut RUNGJINDAMAIb, Jariya SAKAYAROJb, Nattawut BOONYUENb, Sayanh SOMROTHIPOLd, Mohamed A. ABDEL-WAHABa & Ka-Lai PANGc aBotany and Microbiology Department, College of Science, King Saud University, Riyadh, 1145, Saudi Arabia; email: [email protected] bFungal Diversity Laboratory (BFBD), National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Phahonyothin Road, Khlong Nueng, Khlong Luang, Pathum Thani, Thailand cInstitute of Marine Biology and Center of Excellence for the Oceans, National Taiwan Ocean University, 2 Pei-Ning Road, Keelung 20224, Taiwan (R.O.C.) dMicrobe Interaction Laboratory (BMIT), National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Phahonyothin Road, Khlong Nueng, Khlong Luang, Pathum Thani, Thailand Abstract – The taxonomic position of the marine fungi referred to the TBM clade is re-evaluated along with the marine species Chaetosphaeria chaetosa, and the terrestrial asexual genus Falcocladium. Phylogenetic analyses of DNA sequences of two ribosomal nuclear loci of the above taxa and those previous recognized as the TBM clade suggest that they form a distinct clade amongst the Hypocreales, Microascales, Savoryellales, Coronophorales and Melanosporales in the Hypocreomycetidae. Four well-supported subclades in the “TBM clade” are discerned including: 1) the Juncigena subclade, 2) the Etheirophora and Swampomyces s. s. subclade, 3) the Falcocladium subclade and 4) the Torpedospora subclade. Chaetosphaeria chaetosa does not group in the Chaetosphaeriales but together with Swampomyces aegyptiacus and S. -

AR TICLE Recommendations for Competing Sexual-Asexually Typified
IMA FUNGUS · 7(1): 131–153 (2016) doi:10.5598/imafungus.2016.07.01.08 Recommendations for competing sexual-asexually typified generic names in ARTICLE Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales) Martina Réblová1, Andrew N. Miller2, Amy Y. Rossman3*, Keith A. Seifert4, Pedro W. Crous5, David L. Hawksworth6,7,8, Mohamed A. Abdel-Wahab9, Paul F. Cannon8, Dinushani A. Daranagama10, Z. Wilhelm De Beer11, Shi-Ke Huang10, Kevin D. Hyde10, Ruvvishika Jayawardena10, Walter Jaklitsch12,13, E. B. Gareth Jones14, Yu-Ming Ju15, Caroline Judith16, Sajeewa S. N. Maharachchikumbura17, Ka-Lai Pang18, Liliane E. Petrini19, Huzefa A. Raja20, Andrea I Romero21, Carol Shearer2, Indunil C. Senanayake10, Hermann Voglmayr13, Bevan S. Weir22, and Nalin N. Wijayawarden10 1Department of Taxonomy, Institute of Botany of the Academy of Sciences of the Czech Republic, Průhonice 252 43, Czech Republic 2Illinois Natural History Survey, University of Illinois, Champaign, Illinois 61820, USA 3Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331, USA; *corresponding author e-mail: amydianer@ yahoo.com 4Ottawa Research and Development Centre, Biodiversity (Mycology and Microbiology), Agriculture and Agri-Food Canada, 960 Carling Avenue, Ottawa, Ontario K1A 0C6 Canada 5CBS-KNAW Fungal Biodiversity Institute, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands 6Departamento de Biología Vegetal II, Facultad de Farmacia, Universidad Complutense, Plaza de Ramón y Cajal s/n, Madrid 28040, Spain 7Department of Life Sciences,