Evaluation of Citrate Metabolism in Oenococcus Oeni and Lactobacillus Plantarum
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

BJCP Exam Study Guide
BJCP BEER EXAM STUDY GUIDE Last Revised: December, 2017 Contributing Authors: Original document by Edward Wolfe, Scott Bickham, David Houseman, Ginger Wotring, Dave Sapsis, Peter Garofalo, Chuck Hanning. Revised 2006 by Gordon Strong and Steve Piatz. Revised 2012 by Scott Bickham and Steve Piatz. Revised 2014 by Steve Piatz Revised 2015 by Steve Piatz Revised 2017 by Scott Bickham Copyright © 1998-2017 by the authors and the BJCP CHANGE LOG January-March, 2012: revised to reflect new exam structure, no longer interim May 1, 2012: revised yeast section, corrected T/F question 99 August, 2012: removed redundant styles for question S0, revised the additional readings list, updated the judging procedure to encompass the checkboxes on the score sheet. October 2012: reworded true/false questions 2, 4, 6, 8, 13, 26, 33, 38, 39, 42, and 118. Reworded essay question T15. March 2014: removed the Exam Program description from the document, clarified the wording on question T13. October 2015: revised for the 2015 BJCP Style Guidelines. February, 2016: revised the table for the S0 question to fix typos, removed untested styles. September-October, 2017 (Scott Bickham): moved the BJCP references in Section II.B. to Section I; incorporated a study guide for the online Entrance exam in Section II; amended the rubric for written questions S0, T1, T3, T13 and T15; rewrote the Water question and converted the rubrics for each of the Technical and Brewing Process questions to have three components; simplified the wording of the written exam questions’ added -
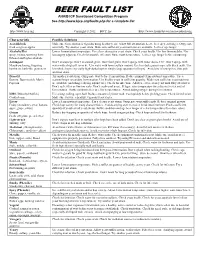
BJCP Beer Fault List
BBEEEERR FFAAUULLTT LLIISSTT AHA/BJCP Sanctioned Competition Program See http://www.bjcp.org/faults.php for a complete list http://www.bjcp.org Copyright © 2012 — BJCP, Inc. http://www.homebrewersassociation.org Characteristic Possible Solutions Acetaldehyde Make sure fermentation is vigorous using healthy yeast. Allow full attenuation. Leave beer on yeast longer. Oxygenate fresh cut green apples wort fully. Try another yeast strain. Make sure sufficient yeast nutrients are available. Let beer age longer. Alcoholic/Hot Lower fermentation temperature. Use a less attenuative yeast strain. Check yeast health. Use less fermentables. Use spicy, vinous, warming from less sugary adjuncts. Check for possible infection. Raise mash temperature. Let beer age longer before consuming. Ethanol and higher alcohols Astringent Don’t oversparge. Don’t overcrush grain. Don’t boil grain. Don’t sparge with water above 170°. Don’t sparge with Mouth-puckering, lingering water with a high pH (over 6). Use water with lower sulfate content. Use less dark grains (especially black malt). Use harshness, husk-like graininess less whole hops (especially high-alpha hops or simply large quantities of hops). Avoid use of raw spices, fruit pith and fruit skins. Diacetyl Try another yeast strain. Oxygenate wort before fermentation. Reduce primary fermentation temperature. Use a Buttery, Butterscotch, Movie warmer/longer secondary fermentation. Use healthy yeast in sufficient quantity. Make sure sufficient yeast nutrients Popcorn are available (including reducing adjunct use). Check for infection. Allow beer to rest on yeast until fully attenuated. Don’t rack, filter or fine too early. Don’t crash-cool yeast. If lager, raise temperature for a diacetyl rest at end of fermentation. -

Shropshire Tales Ad OL EMAIL
FREE Issue 8 Spring 19 Inside... Mild in May Passport Pub, Club and Brewery of the Year Results CAMRA West Midlands Regional Awards 2018 Pub and Brewery News Shropshire's Real Heritage Pubs www.tes.camra.org.uk Telford & East Shropshire CAMRA Offi cial magazine of Telford & East Shropshire and Shrewsbury & www.saws.camra.org.uk Shrewsbury & West Shropshire CAMRA West Shropshire branches of CAMRA Editor's Welcome Main Contents Hello and welcome to Issue 8 of A word from T.E.S CAMRA Chairman 4 A word from S.W.S CAMRA Chairman 4 So the votes have been cast and counted A word from our Market Drayton sub-branch 5 and we now have the results from both Pub of the Season - Spring2018 5 Shropshire branches for Pub, Club and Brewery News 7-8 Brewery of the Year for each area, see the results later in this issue. Pub News 10 CAMRA West Midlands Regional Awards 12-13 There has been some exciting news from 2018 West Midlands CAMRA as our region will TES Pub, Club & Brewery of the Year 15 be hosting the Great British Beer Festival Results Winter in Birmingham from 2020 until Mild in May 17-24 2022. This is great for us as a region SWS Pub & Club of the Year Results 26-27 and more details will be announced Notices & Emails 27 throughout the coming year. Awards presented to Shropshire pubs 28-29 Finally we are pleased to announce that & breweries Mild in May has returned to Shropshire Shropshire's Real Heritage Pubs - 30-31 this year and you can fi nd your Mild in Loggerheads, Shrewsbury May Passport in the middle of this issue Beer Festivals 2019 34 of Shropshire TAles. -

Cervejas Ruivas Para Verdadeiros Experts!
#05 quadrimestral abril ∞ julho ENTREVISTA Bruno Aquino em busca da 3.90 cultura cervejeira € LJUBOMIR STANISIC O cozinheiro que prefere cerveja artesanal 2019 . PORTUGAL CONTINENTAL 2019 . PORTUGAL CONTINENTAL TENDÊNCIAS Cerveja sem álcool conquista mercado CERVEJA E ARTE Vhils desenha coluna de cerveja CERVEJAS RUIVAS PARA VERDADEIROS EXPERTS! BOHEMIA | 1163 | MAJOR ROÇADAS | CERVETECA | PETISCARIA ST.º ANTÓNIO | PURISTA BARBIÈRE POST SCRIPTUM | LUPUM | ROTA DAS TAPAS | CERVEJÃO | MUSA | DEFEITOS: OXIDAÇÃO | NOVIDADES A TUA NOVA CERVEJA purple EDITORIAL proprietário e editor PurpleSummer Media & Events, Unip. Lda Lopes Henriques NPCpurple 513 091 378 • Capital Social € 5 000 A TUA NOVA CERVEJA Rua Manuel da Silva, n.º 2, 1º Frente 2700-552 Amadora | Portugal T. +351 211 352 336 diretora editorial Susana Marvão [email protected] T. +351 938 762 939 diretora executiva Maria Helena Duarte [email protected] T. +351 969 105 600 assessora da direção publicidade e assinaturas Cristina Ribeiro [email protected] departamento comercial Célia Borges [email protected] T. +351 912 411 317 Isabel Vieira [email protected] T. +351 962 943 695 redatores António Mendes Nunes, Aquiles Pinto, Augusto Lopes, Bruno Aquino, Carlos Ramos, Cláudia Pinto, João Pereira Santos, João Durães, SUSANA MARVÃO Manuel Baiôa, Mafalda Freire, Maria Helena Duarte, diretora editorial Paulo Pimenta, Pedro Moura, Sérgio Costa Lopes, Susana Marvão, Tito Santos fotografia Carlos Figueiredo, Ernesto Fonseca, D.R. Paixão Pela Cerveja tem o apoio da Associação Cervejeiros de Portugal impressão LusoImpress, S.A. R. Venceslau Ramos, 28 4430-929 Avintes VNG À conquista da tiragem 3000 exemplares maturidade do mercado distribuição VASP, MLP – Media Logistics Park Quinta do Grajal, Venda Seca 2739-511 Agualva Cacém Há demasiadas cervejas no mercado? À medida que vamos falando com cada vez Registo ERC | 127067 mais produtores de cerveja, a opinião parece alastrar-se não em forma de inter- Depósito Legal | 435346/17 rogação mas de ponto final. -

Brewsletter 02
M RANGE FOA RS THE FOAM RANGERS EST. 1981 BBrreewwsslleetttteerr UUrrqquueellll H O N M TO It's Before 9 A.M. Somewhere! February 2011 - Volume 31 Pints - Issue 2 EBR US EW CLUB - HO DeFalco’s Rocked In this Issue: By “Leaky Wicks” Minutes & MCAB 2 Scandal. By Bev Blackwood & Steve Moore Febrewarys DeFalco’s owner Scott Birdwell is on the Past 3 verge of ousting the Foam Rangers from the shop premises due to the influence of Glory Days 4 the”leaky wicks” scandal. The Foam Rangers, with their Many leaky wicks lead to a 9 A.M. Already? 5 preponderance of male members and major problem at DeFalco’s. the massive amounts of beer available outhouse!” She glares across to the at meetings often find themselves at the homebrew shop “Those boys be leakin’ on Incriminating mercy of the “one holer” bathroom. “God my doorstep and I am going to start kickin’ Evidence 6-7 forbid a woman gets in there!” Bemoans their behinds!” an unidentified Ranger, “We would all be Birdwell, in the throes of trying to move his Snowflakes & out the door!” Indeed, that seems to be the shop isn’t happy about the situation either. major issue (as it were) as Foam Rangers Plain Ol’ Flakes 9 “You know, they could cross their legs for a “leak” in (and on) the surrounding premises. bit” he gripes. “But NO...” He’s been hit with “I come in Saturday mornings after a penalties and threats of being cut off from Fred Fights For meeting” complains the hairdresser Mastercard and Visa, who are responding to Beer! 10 next door, “and it smells like my mama’s complaints from the surrounding merchants. -

How to Judge Beer Thomas Barnes C
How to Judge Beer Thomas Barnes c. 2012 of this guide. Introduction This guide has two purposes. First, it is intended to help you become a better beer judge by teaching you how to judge What Isn’t In This Guide beer and how to fill out scoresheets in BJCP sanctioned While I’ve written this manual for novice judges, it competitions. Second, it is intended to help you apply your assumes some familiarity with the BJCP, its style guidelines and judging experience to get a Recognized or better score (60% or its competition procedures. Before you read this guide, you better) on the BJCP Tasting Exam. should have read (or at least skimmed) the following documents: Much of the work in this guide is adapted from previous BJCP Judge Procedures Manual works by Steve Antoch, Scott Bickham, Al Boyce, Mark (http://www.bjcp.org/judgeprocman.php) Emiley, Peter Garafolo, Randy Mosher, Kevin Pratt, Gordon BJCP Sanctioned Competition Handbook Strong, David Teckham, and others. See the References section (http://www.bjcp.org/docs/SCPCompHdbk.pdf) for details. Judge Instructions Special thanks to Kevin Pratt for reviewing earlier versions (http://www.bjcp.org/docs/SCP_JudgeInstructions.pdf) Judge Procedures Manual (http://www.bjcp.org/judgeprocman.php) Glassware BJCP Membership Guide/First Time Judge Packet The shape of your drinking vessel, and the type of (http://www.bjcp.org/docs/First_Time_Judge_Packet.pdf) material it’s made from, can affect your sensory perceptions. Glasses shaped like a truncated inverted cone, like the You should also have a passing familiarity with the style typical 12 oz. -

Baron Mind 200005A.Pub
May 2000 From the Presidents Desk... HI Everyone, and help us first timers over any hurdle. The event almost went off without a prob- Well we just completed a very busy lem. All things in life should be so easy. time with WORLD BEER CUP, what a GREAT FESTIVAL IT WAS, FUN At the end of the final judging day we all WAS HAD BY ALL. The Beers we sat down to a taste of the world's most ex- got to sample were excellent, most pensive "Beer", SAM ADAMS MILLE- were great examples of their style. NIUM. It was a very different style of beverage, closer in taste to JACK I personally was very impressed with this DANIELS than to beer, but it was worth contest and how it ran. The folks from the trying since it was free. I personally can't AHA are tops at this type of event, willing justify the price of $170.00 for any bottle to answer any question, solve any problem, (Continued on page 2) Upcoming May Meeting The May monthly meeting will be held at Events Clifford’s, 10448 W. Forest Home Ave., Hales Corners, at 7:30 PM on Wednesday, May 24th. Meetings The usual $5.00 meeting fee will be assessed. May 24th: Marianne Gruber from Briess Malt Our speaker will be Marianne Gruber from Briess Malting Company www.briess.com June 28th: Homebrew night; • Mike Rice will lead a Beer Fault Featured beers will be a variety of Pale Ales. evaluation session. We expect to be serving: Bass, Young's Ram Rod, • Free admission if you bring a 6- Fuller's London Pride, Lakefront Pale Ale, and Si- pack of homebrew (or equivalent) erra Nevada Pale Ale July 26th: Tentative - Mexican Beers Aug 23rd: Tentative - Weiss Beers Membership Information: Other Club Events The Beer Barons of Milwaukee is open to any- May 20th, Brewing Party at Road America, one 21 years of age or older. -

Common Homebrew Off-Flavors & How to Fix Them
Common Homebrew Off-Flavors & How to Fix Them 1. Skunk Skunky and rubbery flavors or aromas in homebrew can be signs of “light-struck” homebrew. This quality is typically never desired in any style of beer. Beer becomes light-struck when the isohumulone bittering compounds in hops come into contact and react with specific wavelengths of light. Skunky aroma can also be caused by using Cluster hops for late boil additions. To prevent light-stricken beer in the future, use brown bottles when packaging and store bottles out of the light. In some instances, it only takes a matter of seconds for the reaction to occur and start instilling the undesirable skunk off-flavor. 2. Solvent Also described as “hot” or high in fusel alcohol qualities, solvent-y brew is often characterized by an unpleasant heat from alcohol, and in some cases a burning sensation on the palate. It can be perceived in flavor, aroma and mouth feel. In some stronger beer styles, perceivable alcohol is expected at certain levels. “Hot” beer can be caused by fermenting too warm, not using enough healthy yeast or simply the beer being young. To address solvent-y beers, first try simply setting the flawed beer aside and giving it time. Sometimes beers need longer aging periods to round out flavors and aromas. If time does not fix this issue, you can take measures to prevent this from happening next time you brew the recipe. Depending on what your brew day notes dictate, consider cooler fermentation temperatures, more viable yeast cells or even a different yeast strain altogether. -

UNIVERSITY of CALIFORNIA Santa Barbara Invisible Hooves
UNIVERSITY OF CALIFORNIA Santa Barbara Invisible Hooves: Markets and the Environment in the History of American and Transnational Cattle Ranching, 1867-2017 A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in History by Timothy Amund Paulson Committee in charge: Professor Peter Alagona, Chair Professor Nelson Lichtenstein Professor Erika Rappaport Professor Simone Pulver September 2017 The dissertation of Tim Paulson is approved. _____________________________________________ Simone Pulver _____________________________________________ Erika Rappaport _____________________________________________ Nelson Lichtenstein _____________________________________________ Peter Alagona, Committee Chair September 2017 © Tim Paulson, 2017. iii Curriculum Vitae Education Ph.D. University of California, Santa Barbara, Department of History (2017). M.A. University of California, Santa Barbara, Department of History (2013). B. A. University of Victoria, Department of History (2010). Publications Peter S. Alagona and Tim Paulson, “From the Classroom to the Countryside: The University of California’s Natural Reserve System and the Role of Field Stations in American Academic Life,” Landscape and the Academy: Dumbarton Oaks Garden and Landscape Studies Series (Cambridge, MA: Harvard University Press, Forthcoming 2017). Tim Paulson, “From ‘Knife Men’ to ‘Streamlining with Curves’: Structure, Skill, and Gender in British Columbia’s Meat-Packing Industry,” BC Studies 193 (Spring 2017), 115-145. Peter S. Alagona, Tim Paulson, Andrew B. Esch and Jessica Marter-Kenyon, “Population and Land Use,” Ecosystems of California, Hal Mooney and Erika Zavaleta, eds. (Oakland, CA: University of California Press, 2016). Book Review by Tim Paulson: Jack Stauder, The Blue and the Green: A Cultural Ecological History of an Arizona Ranching Community (Reno, NV: University of Nevada Press, 2016), The Public Historian 39, no. -

The Complete Beer Fault Guide V. 1.4 © Thomas Barnes, 2011-2013 Permission Is Granted to Reprint This Guide for Personal Or Non-Profit Use
The Complete Beer Fault Guide v. 1.4 © Thomas Barnes, 2011-2013 Permission is granted to reprint this guide for personal or non-profit use Introduction * It is typically produced as a precursor to ethanol This guide is a summary of my research into sensory produced during fermentation: glucose is metabolized into characteristics of beer. Information has been gleaned from a pyruvic acid which is then converted to acetaldehyde and then to number of sources, notably George Fix’s Principles of Brewing ethanol. During fermentation some acetaldehyde escapes from Science, 2nd edition, but also a variety of online and academic the yeast cell. During the final phases of fermentation, the yeast sources. Even so, it is a work in progress. Eventually, it will scavenges free acetaldehyde and finishes converting it. include complete information on every sensory descriptor listed * In young beer or fermenting wort, acetaldehyde levels on the Meilgaard Flavor Wheel, as well as other sensory range from 20-40 mg/l, decreasing to 8-10 mg/l in finished beer. descriptors discovered by fermentation scientists. * Oxidation of finished beer might convert ethanol back Sensory descriptors are listed in alphabetical order. Note into acetaldehyde. In this case, acetaldehyde is usually that some descriptors don’t necessarily agree with information accompanied by other oxidation and/or age-related defects. published by the BJCP. * The combination of bacterial action and oxidation can reduce acetaldehyde to acetic acid (vinegar) due to the chemical reaction of ethanol and acetaldehyde. In this case, acetaldehyde 5.2™ is usually accompanied by acetic acid and other contamination Detected In: Flavor, mouthfeel. -

Traditional Fermented Food and Beverages for Improved Livelihoods Traditional the Diversification Booklets Are Not Intended to Be Technical ‘How to Do It’ Guidelines
ISSN 1810-0775 Traditional ferme nted food and beve rages for imp roved livelihoods )$2'LYHUVLÀFDWLRQERRNOHW Diversification booklet number 21 al fe Tradition rmented be food and verages for improved livelihoods Elaine Marshall and Danilo Mejia Rural Infrastructure and Agro-Industries Division Food and Agriculture Organization of the United Nations Rome 2011 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. ISBN 978-92-5-107074-1 All rights reserved. FAO encourages reproduction and dissemination of material in this information product. Non-commercial uses will be authorized free of charge, upon request. Reproduction for resale or other commercial purposes, including educational purposes, may incur fees. Applications for permission to reproduce or disseminate FAO copyright materials, and all queries concerning rights and licences, should be addressed by e-mail to [email protected] or to the Chief, Publishing Policy and Support Branch, Office of Knowledge Exchange, Research and Extension, FAO, Viale delle Terme di Caracalla, 00153 Rome, Italy. -

Shropshire Tales Issue 8 Spring 2019
FREE Issue 8 Spring 19 Inside... Mild in May Passport Pub, Club and Brewery of the Year Results CAMRA West Midlands Regional Awards 2018 Pub and Brewery News Shropshire's Real Heritage Pubs www.tes.camra.org.uk Telford & East Shropshire CAMRA Offi cial magazine of Telford & East Shropshire and Shrewsbury & www.saws.camra.org.uk Shrewsbury & West Shropshire CAMRA West Shropshire branches of CAMRA Editor's Welcome Main Contents Hello and welcome to Issue 8 of A word from T.E.S CAMRA Chairman 4 A word from S.W.S CAMRA Chairman 4 So the votes have been cast and counted A word from our Market Drayton sub-branch 5 and we now have the results from both Pub of the Season - Spring2018 5 Shropshire branches for Pub, Club and Brewery News 7-8 Brewery of the Year for each area, see the results later in this issue. Pub News 10 CAMRA West Midlands Regional Awards 12-13 There has been some exciting news from 2018 West Midlands CAMRA as our region will TES Pub, Club & Brewery of the Year 15 be hosting the Great British Beer Festival Results Winter in Birmingham from 2020 until Mild in May 17-24 2022. This is great for us as a region SWS Pub & Club of the Year Results 26-27 and more details will be announced Notices & Emails 27 throughout the coming year. Awards presented to Shropshire pubs 28-29 Finally we are pleased to announce that & breweries Mild in May has returned to Shropshire Shropshire's Real Heritage Pubs - 30-31 this year and you can fi nd your Mild in Loggerheads, Shrewsbury May Passport in the middle of this issue Beer Festivals 2019 34 of Shropshire TAles.