A New Species of Alesa (Riodinidae: Eurybiini) from Eastern Ecuador
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera: Papilionoidea) Q ⇑ Marianne Espeland A,B, , Jason P.W
Molecular Phylogenetics and Evolution 93 (2015) 296–306 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Ancient Neotropical origin and recent recolonisation: Phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea) q ⇑ Marianne Espeland a,b, , Jason P.W. Hall c, Philip J. DeVries d, David C. Lees e, Mark Cornwall a, Yu-Feng Hsu f, Li-Wei Wu g, Dana L. Campbell a,h, Gerard Talavera a,i,j, Roger Vila i, Shayla Salzman a, Sophie Ruehr k, David J. Lohman l, Naomi E. Pierce a a Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA b McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Powell Hall, 2315 Hull Road, Gainesville, FL 32611, USA c Department of Systematic Biology-Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560-127, USA d Department of Biological Sciences, University of New Orleans, 2000 Lake Shore Drive, New Orleans, LA 70148, USA e Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK f Department of Life Science, National Taiwan Normal University, Taipei, Taiwan g The Experimental Forest, College of Bio-Resources and Agriculture, National Taiwan University, Nantou, Taiwan h Division of Biological Sciences, School of Science, Technology, Engineering & Mathematics, University of Washington Bothell, Box 358500, 18115 Campus Way NE, Bothell, WA 98011-8246, USA i Institut de Biologia Evolutiva (CSIC-UPF), Pg. Marítim de la Barceloneta 37, 08003 Barcelona, Spain j Faculty of Biology & Soil Science, St. -

Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province
Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, and North Carolina, NAPPC South Carolina, Tennessee Table of CONTENTS Why Support Pollinators? 4 Getting Started 5 Central Appalachian Broadleaf Forest 6 Meet the Pollinators 8 Plant Traits 10 Developing Plantings 12 Far ms 13 Public Lands 14 Home Landscapes 15 Bloom Periods 16 Plants That Attract Pollinators 18 Habitat Hints 20 This is one of several guides for Check list 22 different regions in the United States. We welcome your feedback to assist us in making the future Resources and Feedback 23 guides useful. Please contact us at [email protected] Cover: silver spotted skipper courtesy www.dangphoto.net 2 Selecting Plants for Pollinators Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Ecological Region of the Central Appalachian Broadleaf Forest Coniferous Forest Meadow Province Including the states of: Maryland, Pennsylvania, Virginia, West Virginia And parts of: Georgia, Kentucky, North Carolina, South Carolina, Tennessee a nappc and Pollinator Partnership™ Publication This guide was funded by the National Fish and Wildlife Foundation, the C.S. Fund, the Plant Conservation Alliance, the U.S. Forest Service, and the Bureau of Land Management with oversight by the Pollinator Partnership™ (www.pollinator.org), in support of the North American Pollinator Protection Campaign (NAPPC–www.nappc.org). Central Appalachian Broadleaf Forest – Coniferous Forest – Meadow Province 3 Why support pollinators? In theIr 1996 book, the Forgotten PollInators, Buchmann and Nabhan estimated that animal pollinators are needed for the reproduction “ Farming feeds of 90% of flowering plants and one third of human food crops. -

Un Nouvel Eurybiini Du Genre Alesa De Guyane Française (Lepidoptera, Riodinidae)
Bulletin de la Société entomologique de France, 120 (4), 2015 : 453-456. Un nouvel Eurybiini du genre Alesa de Guyane française (Lepidoptera, Riodinidae) par Christian BRÉVIGNON Villa A7, Rochambeau, F – 97351 Matoury <[email protected]> Résumé. – Une nouvelle espèce du genre Alesa Doubleday, 1847, originaire de Guyane française, Alesa humilis n. sp., est décrite et comparée avec deux espèces proches, A. telephae (Boisduval, 1836) et A. amethystina Gallard & Fernandez, 2015. Abstract. – A new Eurybiini of the genus Alesa from French Guiana (Lepidoptera, Riodinidae). A new species of the genus Alesa Doubleday, 1847, from French Guiana, Alesa humilis n. sp., is described and compared with two closely allied species, A. telephae (Boisduval, 1836) and A. amethystina Gallard & Fernandez, 2015. Keywords. – Riodininae, new species, taxonomy, Guiana shield. _________________ Le genre Alesa Doubleday, 1847, a récemment fourni matière à description de nouveaux taxa (SALAZAR & CONSTANTINO, 2007 ; HALL & AHRENHOLZ, 2010 ; GALLARD & FERNANDEZ, 2015). Un spécimen mâle de la collection L. & C. Brévignon avait été mis de côté depuis de nombreuses années en vue d’un examen plus approfondi. Les motifs alaires et la structure des genitalia permettent de différencier ce spécimen des taxa décrits jusqu’alors, et particulièrement d’Alesa telephae (Boisduval, 1836) et d’A. amethystina Gallard & Fernandez, 2015. L’objet de cet article est la description de cette nouvelle espèce. Alesa humilis n. sp. (fig. 1-2) HOLOTYPE : ♂, Guyane française, Roura, RN 2, PK 35, 14.X.1989, collection L. & C. Brévignon (Matoury, Guyane française). Description du mâle. – Longueur de l’aile antérieure : 19,5 mm. Antennes brun clair, trompe beige, vertex brun, pattes antérieures beiges, abdomen court, légèrement teinté d’orangé face dorsale. -

Revision of the Genera Tiniocellus Péringuey, 1901 and Nitiocellus Gen
Boletín de la Sociedad Entomológica Aragonesa (S.E.A.), nº 47 (2010) : 71‒126. REVISION OF THE GENERA TINIOCELLUS PÉRINGUEY, 1901 AND NITIOCELLUS GEN. N. (COLEOPTERA, SCARABAEIDAE, ONITICELLINI) Tristão Branco Rua de Camões, 788, 2º Dto, P-4000-142 Porto, Portugal − [email protected] Abstract: The taxonomical history of the genus Tiniocellus Péringuey, 1901 and the 10 species-group names that have been associated with it is reviewed, and the reasons that justify the creation of Nitiocellus gen. n. for Oniticellus panthera Boucomont, 1921 and Oniticellus collarti Janssens, 1939, are explained. The taxonomy of the Oniticellini is briefly reviewed and a key is provided for the separation of Tiniocellus and Nitiocellus gen. n. from all the other genera currently ranged in the tribe. The synonymies of Oniticellus variegatus Fåhraeus, 1857 and Oniticellus humilis Gerstaecker, 1871 with Tiniocellus spinipes (Roth, 1851) are confirmed. The Asian Tiniocellus imbellis (Bates, 1891) and the African Tiniocellus setifer (Kraatz, 1895) are rehabilitated as good species. Oniticellus modestus Arrow, 1908 is synonymised with T. imbellis, and Tiniocellus asmarensis Balthasar, 1968 with T. spinipes. Three Afrotropical species, one of them containing two subspecies, are described: T. praetermissus sp. n. from western Africa, T. dolosus sp. n. from eastern, central and western Africa, T. eurypygus sp. n. from South Africa, the nominotypical subspecies from the uplands west of the Drakensberg mountain range, and T. eurypygus transdrakensbergensis ssp. n. from the lowlands east of the same mountain range. Keys are provided to the species and sub- species of Tiniocellus, and to the species of Nitiocellus gen. n. For this study 4,628 specimens were examined, including the name-bearing types of all the species-group names, except that of T. -

Title Lorem Ipsum Dolor Sit Amet, Consectetur Adipiscing Elit
Volume 26: 102–108 METAMORPHOSIS www.metamorphosis.org.za ISSN 1018–6490 (PRINT) LEPIDOPTERISTS’ SOCIETY OF AFRICA ISSN 2307–5031 (ONLINE) Classification of the Afrotropical butterflies to generic level Published online: 25 December 2015 Mark C. Williams 183 van der Merwe Street, Rietondale, Pretoria, South Africa. E-mail: [email protected] Copyright © Lepidopterists’ Society of Africa Abstract: This paper applies the findings of phylogenetic studies on butterflies (Papilionoidea) in order to present an up to date classification of the Afrotropical butterflies to genus level. The classification for Afrotropical butterflies is placed within a worldwide context to subtribal level. Taxa that still require interrogation are highlighted. Hopefully this classification will provide a stable context for researchers working on Afrotropical butterflies. Key words: Lepidoptera, Papilionoidea, Afrotropical butterflies, classification. Citation: Williams, M.C. (2015). Classification of the Afrotropical butterflies to generic level. Metamorphosis 26: 102–108. INTRODUCTION Suborder Glossata Fabricius, 1775 (6 infraorders) Infraorder Heteroneura Tillyard, 1918 (34 Natural classifications of biological organisms, based superfamilies) on robust phylogenetic hypotheses, are needed before Clade Obtectomera Minet, 1986 (12 superfamilies) meaningful studies can be conducted in regard to their Superfamily Papilionoidea Latreille, 1802 (7 evolution, biogeography, ecology and conservation. families) Classifications, dating from the time of Linnaeus in the Family Papilionidae Latreille, 1802 (32 genera, 570 mid seventeen hundreds, were based on morphology species) for nearly two hundred and fifty years. Classifications Family Hedylidae Guenée, 1858 (1 genus, 36 species) based on phylogenies derived from an interrogation of Family Hesperiidae Latreille, 1809 (570 genera, 4113 the genome of individual organisms began in the late species) 20th century. -

Devries, P.J., B.C. Cabral, C.M. Penz. 2004
N. 102 May 31, 2004 ~ C/) in Biology and Geology :J ~ C/) Z :J ~ 0 ~ The early stages of Apodemia paucipuncta U (Riodinidae): myrmecophily, ~ •.......• a new caterpillar ant-organ ........l and consequences for classification ~ :J By Pi. De Vries :J ~ Center for Biodiversity Studies Milwaukee Public Museum ~ ~ ~ 800 West Wells Street Milwaukee, WI 53233, USA ~ Berites c. Cabral ~ Departamento de Zoologia ~ Universidade de Brasilia P.O. Box 04525 ~ ~ Brasilia, Distrito Federal 70919-970, Brazil :J Carla M. Pen: -< Department of Invertebrate Zoology Z Milwaukee Public Museum 800 West Wells Street, ~ Milwaukee, WI 53233, USA ........l 0 •.......• Milwaukee Public ~ U MUSEUM Milwaukee Public Museum Contributions in Biology and Geology Paul Mayer, Editor Reviewer for this Publication: Andre Victor Lucci Freitas, Museu de Historia Natural, Instituto de Biologia, Universidade Estadual de Campinas This publication is priced at $6.00 and may be obtained by writing to the Museum Shop, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, WI 53233. Orders must include $3.00 for shipping and handling ($4.00 for foreign destinations) and must be accompanied by money order or check drawn on U.S. bank. Money orders or checks should be made payable to the Milwaukee Public Museum, Inc. Wisconsin residents please add 5% sales tax. ISBN 0-89326-215-3 ©2004 Milwaukee Public Museum, Inc. Abstract The early stages of Apodemia paucipuncta are described for the first time. This species forms symbiotic associations with Crematogaster ants in central Brazil, and possesses four sets of ant-organs: tentacle nectary organs, vibratory papillae, balloon setae and for the first time in the Riodinidae, a cervical gland that is used in myrmecophily. -
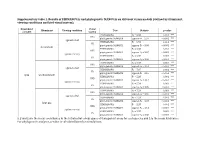
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

Phylogeny of the Riodinid Butterfly Subtribe Theopeina (Lepidoptera: Riodinidae: Nymphidiini)
Systematic Entomology (2002) 27, 139±167 Phylogeny of the riodinid butterfly subtribe Theopeina (Lepidoptera: Riodinidae: Nymphidiini) JASON P. W. HALL Department of Systematic Biology±Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC, U.S.A. Abstract. The almost exclusively Neotropical butterfly family Riodinidae is poorly represented in both ecological and systematic studies of Lepidoptera. A comparative morphological study of all seventy-five species in subtribe Theopeina (tribe Nymphidiini) yielded 104 characters, predominantly from wing pattern, male and female genitalia, and abdominal structures. All morphological char- acters and adults representing the range of wing pattern variation are illustrated. Phylogenetic analysis of the data produced a large number of most parsimonious cladograms, but the strict consensus of these, both when using equal weights and after successive weighting, is well resolved and the majority of terminal clades have high character and branch support. Theopeina is found to consist of five mono- phyletic genera, Protonymphidia, Archaeonympha, Calicosama, Behemothia and Theope ( Parnes and Dinoplotis), with the largest genus Theope containing thirteen monophyletic species groups, which are delineated to facilitate a discussion of broad evolutionary patterns in this morphologically diverse subtribe. Introduction family upon which to trace major evolutionary adaptations or test competing hypotheses. Riodinidae are unique among butterflies in being almost The first higher classification of Riodinidae was exclusively confined to a single biogeographical region, attempted by Bates (1868), who recognized three subfamilies the Neotropics, where approximately 1300 species or 95% of and several further divisions based solely on characters of the familial diversity occurs, and where it constitutes about wing venation, antennae and palpi. -

Law Breaker Or Nature Lover?
NEWSLETTER OF THE NC NATIVE PLANT SOCIETY Native Plant News Summer 2020 Julie Higgie, editor Vol. 18, Issue 2 INSIDE: Law Breaker or Nature Lover? P2 President’s You Be the Judge! Report By Julie Higgie, editor P4 Pollinators & Native Plants ews Flash! Life in quarantine N took a strange turn this year P6 Chlorofiends! when Don and I moved to a 55+ neighborhood right during the state P8 Seed Starter! lockdown. Talk about weird and scary. But we had no choice, as we P10 Scholar News had signed papers for this new P11 Good Smells! home last fall. So, we packed boxes and away we went. P12 Member Now we are on a property in the Spotlight same small town, Mooresville, over- looking a small forest that we don’t MISSION have to take care of. Yay! STATEMENT: As most of my friends know, I am an avid, lifelong naturalist by avoca- Our mission is to tion. The home from which we just moved sits on a very steep, Lake promote the en- Norman waterside property certified as a Native Plant Habitat, a Wild- joyment and con- life Habitat, and a pitstop on the Butterfly Highway. It’s full of plants servation of obtained from plant sales, Society auctions, and UNC-Charlotte Bo- tanical Gardens, as well as the Spring Wildflower Pilgrimage in the North Carolina’s Great Smoky Mountains National Park. native plants and their habitats Fortunately, we were able to sell that woodsy paradise to a woman through educa- who is also an avid gardener, but has no fear of falling down the steep hill as we have done too many times. -

This Article Appeared in a Journal Published by Elsevier. the Attached
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Arthropod Structure & Development 40 (2011) 122e127 Contents lists available at ScienceDirect Arthropod Structure & Development journal homepage: www.elsevier.com/locate/asd The extremely long-tongued Neotropical butterfly Eurybia lycisca (Riodinidae): Proboscis morphology and flower handling Julia A.S. Bauder*, Nora R. Lieskonig, Harald W. Krenn Department of Evolutionary Biology, University of Vienna, Althanstrasse 14, A-1090 Vienna, Austria article info abstract Article history: Few species of true butterflies (Lepidoptera: Papilionoidea) have evolved a proboscis that greatly exceeds Received 17 June 2010 the length of the body. This study is the first to examine the morphology of an extremely long butterfly Accepted 22 November 2010 proboscis and to describe how it is used to obtain nectar from flowers with very deep corolla tubes. The proboscis of Eurybia lycisca (Riodinidae) is approximately twice as long as the body. It has a maximal Keywords: length of 45.6 mm (mean length 36.5 mm Æ 4.1 S.D., N ¼ 20) and is extremely thin, measuring only about Mouthparts 0.26 mm at its maximum diameter. -

Papilionoidea (Butterfly & Skipper) Species List
Papilionoidea (Butterfly & Skipper) Species List Higher Classification1 Kingdom: Animalia, Phylum: Arthropoda, Class: Insecta, Order: Lepidoptera, Superfamily: Papilionoidea Family (F:), Subfamily (sF:) and Tribe (T:) Scientific Name1 English Name1 F: Hesperiidae (Skippers) sF: Eudaminae (Spreadwing Skippers) Astraptes anaphus annetta Yellow-tipped Flasher Central American Banded- Autochton vectilucis Skipper Urbanus pronus Pronus Longtail sF: Hesperiinae (Grass Skippers) T: Anthoptini Synapte salenus salenus Salenus Faceted-Skipper T: Calpodini Calpodes cf. ethlius Brazilian Skipper Talides alternata Alternate Ruby-eye T: Hesperiini Hylephila cf. phyleus phyleus Fiery Skipper Poanes inimica Yellow-stained Skipper Poanes cf. zabulon Hobomok Skipper T: Moncini Halotus angellus Angellus Skipper Lerema accius Clouded Skipper Remella rita Rita's Remella sF: Heteropterinae (Skipperlings) Dalla lethaea Schaus' Skipperling sF: Pyrginae (Spread-wing Skippers) T: Achlyodidini Doberes anticus Dark Doberes T: Carcharodini Noctuana lactifera lactifera Cryptic Skipper T: Erynnini Mylon cf. maimon Common Mylon F: Lycaenidae (Gossamerwings) sF: Theclinae (Hairstreaks) T: Eumaeini (Hairstreaks) Contrafacia bassania White-etched Hairstreak F: Nymphalidae (Brushfoots) sF: Apaturinae (Emperors) Doxocopa cyane mexicana Mexican Emperor Doxocopa laurentia cherubina Turquoise Emperor sF: Biblidinae (Exotic Brushfoots) T: Callicorini Diaethria anna anna Anna’s Eighty-eight Diaethria astala astala Astala Eighty-eight Diaethria clymena marchalii Widespread Eighty-eight -

White Wood Aster (Eurybia Divaricata) in Ontario
Photo: Rob Tervo White Wood Aster (Eurybia divaricata) in Ontario Ontario Recovery Strategy Series 2019 Ministry of the Environment, Conservation and Parks About the Ontario Recovery Strategy Series This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada. What is recovery? What’s next? Recovery of species at risk is the process by Nine months after the completion of a recovery which the decline of an endangered, threatened, strategy a government response statement will or extirpated species is arrested or reversed, be published which summarizes the actions that and threats are removed or reduced to improve the Government of Ontario intends to take in the likelihood of a species’ persistence in the response to the strategy. The implementation of wild. recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and What is a recovery strategy? conservationists. Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A For more information recovery strategy outlines the habitat needs To learn more about species at risk recovery in and the threats to the survival and recovery of Ontario, please visit the Ministry of Environment, the species. It also makes recommendations Conservation and Parks Species at Risk webpage on the objectives for protection and recovery, at: www.ontario.ca/speciesatrisk the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation.