Beta-Hydroxy-Beta-Methylbutyrate (HMB)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

Endogenous Metabolites: JHU NIMH Center Page 1
S. No. Amino Acids (AA) 24 L-Homocysteic acid 1 Glutaric acid 25 L-Kynurenine 2 Glycine 26 N-Acetyl-Aspartic acid 3 L-arginine 27 N-Acetyl-L-alanine 4 L-Aspartic acid 28 N-Acetyl-L-phenylalanine 5 L-Glutamine 29 N-Acetylneuraminic acid 6 L-Histidine 30 N-Methyl-L-lysine 7 L-Isoleucine 31 N-Methyl-L-proline 8 L-Leucine 32 NN-Dimethyl Arginine 9 L-Lysine 33 Norepinephrine 10 L-Methionine 34 Phenylacetyl-L-glutamine 11 L-Phenylalanine 35 Pyroglutamic acid 12 L-Proline 36 Sarcosine 13 L-Serine 37 Serotonin 14 L-Tryptophan 38 Stachydrine 15 L-Tyrosine 39 Taurine 40 Urea S. No. AA Metabolites and Conjugates 1 1-Methyl-L-histidine S. No. Carnitine conjugates 2 2-Methyl-N-(4-Methylphenyl)alanine 1 Acetyl-L-carnitine 3 3-Methylindole 2 Butyrylcarnitine 4 3-Methyl-L-histidine 3 Decanoyl-L-carnitine 5 4-Aminohippuric acid 4 Isovalerylcarnitine 6 5-Hydroxylysine 5 Lauroyl-L-carnitine 7 5-Hydroxymethyluracil 6 L-Glutarylcarnitine 8 Alpha-Aspartyl-lysine 7 Linoleoylcarnitine 9 Argininosuccinic acid 8 L-Propionylcarnitine 10 Betaine 9 Myristoyl-L-carnitine 11 Betonicine 10 Octanoylcarnitine 12 Carnitine 11 Oleoyl-L-carnitine 13 Creatine 12 Palmitoyl-L-carnitine 14 Creatinine 13 Stearoyl-L-carnitine 15 Dimethylglycine 16 Dopamine S. No. Krebs Cycle 17 Epinephrine 1 Aconitate 18 Hippuric acid 2 Citrate 19 Homo-L-arginine 3 Ketoglutarate 20 Hydroxykynurenine 4 Malate 21 Indolelactic acid 5 Oxalo acetate 22 L-Alloisoleucine 6 Succinate 23 L-Citrulline 24 L-Cysteine-glutathione disulfide Semi-quantitative analysis of endogenous metabolites: JHU NIMH Center Page 1 25 L-Glutathione, reduced Table 1: Semi-quantitative analysis of endogenous molecules and their derivatives by Liquid Chromatography- Mass Spectrometry (LC-TripleTOF “or” LC-QTRAP). -

Opposing Effects of Dehydroepiandrosterone And
European Journal of Endocrinology (2000) 143 687±695 ISSN 0804-4643 EXPERIMENTAL STUDY Opposing effects of dehydroepiandrosterone and dexamethasone on the generation of monocyte-derived dendritic cells M O Canning, K Grotenhuis, H J de Wit and H A Drexhage Department of Immunology, Erasmus University Rotterdam, The Netherlands (Correspondence should be addressed to H A Drexhage, Lab Ee 838, Department of Immunology, Erasmus University, PO Box 1738, 3000 DR Rotterdam, The Netherlands; Email: [email protected]) Abstract Background: Dehydroepiandrosterone (DHEA) has been suggested as an immunostimulating steroid hormone, of which the effects on the development of dendritic cells (DC) are unknown. The effects of DHEA often oppose those of the other adrenal glucocorticoid, cortisol. Glucocorticoids (GC) are known to suppress the immune response at different levels and have recently been shown to modulate the development of DC, thereby influencing the initiation of the immune response. Variations in the duration of exposure to, and doses of, GC (particularly dexamethasone (DEX)) however, have resulted in conflicting effects on DC development. Aim: In this study, we describe the effects of a continuous high level of exposure to the adrenal steroid DHEA (1026 M) on the generation of immature DC from monocytes, as well as the effects of the opposing steroid DEX on this development. Results: The continuous presence of DHEA (1026 M) in GM-CSF/IL-4-induced monocyte-derived DC cultures resulted in immature DC with a morphology and functional capabilities similar to those of typical immature DC (T cell stimulation, IL-12/IL-10 production), but with a slightly altered phenotype of increased CD80 and decreased CD43 expression (markers of maturity). -

Fluorinated Analogues of Glutamic Acid and Glutamine R
γ-Fluorinated analogues of glutamic acid and glutamine R. Dave, B. Badet, Patrick Meffre To cite this version: R. Dave, B. Badet, Patrick Meffre. γ-Fluorinated analogues of glutamic acid and glutamine. Amino Acids, Springer Verlag, 2003, 24 (3), pp.245-261. 10.1007/s00726-002-0410-9. hal-02002676 HAL Id: hal-02002676 https://hal.archives-ouvertes.fr/hal-02002676 Submitted on 11 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. γ-Fluorinated analogues of glutamic acid and glutamine Review Article 1 2 1 R. Dave , B. Badet , and P. Meffre 1 UMR 7573-C.N.R.S., ENSCP, Paris, France 2 UPR 2301-CNRS, ICSN, Gif-sur-Yvette, France Summary. γ-Fluorinated analogues of glutamic acid and glutamine N-bromosuccinimide; NFSi, N-fluorobenzenesulfonimide; NMR, are compounds of biological interest. Syntheses of such compounds nuclear magnetic resonance; 2-PrOH, isopropanol; PTSA, p- are extensively reviewed in this article. 4-Fluoroglutamic acid toluenesulfonic acid; TCDI, thiocarbonyldiimidazole; TEMPO, was prepared as a mixture of racemic diastereomers by Michael 2,2,6,6-tetramethyl piperidine-1-oxyl; TFA, trifluoroacetic acid. reaction, inverse-Michael reaction or by electrophilic / nucleophilic fluorination. -

Effect of Leucine on Intestinal Absorption of Tryptophan in Rats
Downloaded from https://doi.org/10.1079/BJN19850155 British Journal of Nutrition (1985), 54, 695-703 695 https://www.cambridge.org/core Effect of leucine on intestinal absorption of tryptophan in rats BY CHISAE UMEZAWA, YUKO MAEDA, KANJI HABA, MARIKO SHIN AND KEIJI SANO School of Pharmacy, Kobe-Gakuin University, Nishi-ku, Kobe 673, Japan (Received I7 May 1985 - Accepted 24 June 1985) . IP address: 1. To elucidate the causal relation between leucine and the lowering of hepatic NAD content of rats fed on a leucine-excessive diet (Yamada et aZ. 1979), the effect of leucine on intestinal absorption of tryptophan was 170.106.35.93 investigated. 2. Co-administration of [3H]tryptophan and leucine, with leucine at ten times the level of tryptophan, delayed absorption of L-[side chain 2,3-3H]tryptophan from the digestive tract and incorporation of [3H]tryptophan into portal blood, the liver and a protein fraction of the liver. After 120 min, more than 95% of tryptophan was absorbed whether [3H]tryptophan was administered with or without leucine. , on 3. Co-administration of a mixture of ten essential amino acids, in proportions simulating casein, with 02 Oct 2021 at 04:49:27 [3H]tryptophan markedly delayed absorption of tryptophan from the digestive tract. The addition of supplementary leucine to the amino acid mixture, however, caused no further delay. 4. In rats prefed a leucine-excessive diet for 1 week [3H]tryptophan was absorbed at the same rate as in rats fed on a control diet. 5. The results indicate that competition between tryptophan and leucine for intestinal absorption did not cause lowering of hepatic NAD. -

Electrochemical Studies of Dl-Leucine, L-Proline and L
ELECTROCHEMICAL STUDIES OF DL -LEUCINE, 60 L-PROLINE AND L-TRYPTOPHAN AND THEIR INTERACTION WITH COPPER AND IRON 30 c b A) M. A. Jabbar, R. J. Mannan, S. Salauddin and B. µ a Rashid 0 Department of Chemistry, University of Dhaka, ( Current Dhaka-1000, Bangladesh -30 Introduction -60 In vitro study of the charge transfer reactions coupled -800 -400 0 400 800 with chemical reactions can give important indication of Potential vs. Ag/AgCl (mV) about actual biological processes occurring in human Fig.1. Comparison of the cyclic voltammogram of system. Understanding of such charge-transfer 5.0mM (a) DL -Leucine, (b) Cu-DL -Leucine ion and mechanism will help to determine the effectiveness of (c) [Fe-DL -Leucine] in 0.1M KCl solution at a Pt- nutrition, metabolism and treatment of various biological button electrode. Scan rates 50 mV/s. disorders. In the previous research, the redox behaviour of 40 various amino acids and biochemically important compounds and their charge transfer reaction and their b interaction of metal ions were studied [1,2]. In the present 20 ) a c research, the redox behavior and the charge transfer µΑ kinetics of DL -Leucine, L-Proline and L-Tryptophan in 0 the presence and absence of copper and iron will be investigated. Current ( -20 Experimental A computerized electrochemistry system developed by -40 -800 -400 0 400 800 Advanced Analytics, Virginia, USA, (Model-2040) Potential vs. Ag/AgCl (mV) consisting of three electrodes micro-cell with a saturated Ag/AgCl reference, a Pt-wire auxiliary and a pretreated Fig.2 . Comparison of the cyclic voltammogram of Pt-button working electrode is employed to investigate 5.0mM (a) L-Proline, (b) Cu-L-Proline and (c) Fe-L- Proline in 0.1M KCl solution at a Pt-button different amino acids and metal-amino acid systems. -

Amino Acid Chemistry
Handout 4 Amino Acid and Protein Chemistry ANSC 619 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIES Amino Acid Chemistry I. Chemistry of amino acids A. General amino acid structure + HN3- 1. All amino acids are carboxylic acids, i.e., they have a –COOH group at the #1 carbon. 2. All amino acids contain an amino group at the #2 carbon (may amino acids have a second amino group). 3. All amino acids are zwitterions – they contain both positive and negative charges at physiological pH. II. Essential and nonessential amino acids A. Nonessential amino acids: can make the carbon skeleton 1. From glycolysis. 2. From the TCA cycle. B. Nonessential if it can be made from an essential amino acid. 1. Amino acid "sparing". 2. May still be essential under some conditions. C. Essential amino acids 1. Branched chain amino acids (isoleucine, leucine and valine) 2. Lysine 3. Methionine 4. Phenyalanine 5. Threonine 6. Tryptophan 1 Handout 4 Amino Acid and Protein Chemistry D. Essential during rapid growth or for optimal health 1. Arginine 2. Histidine E. Nonessential amino acids 1. Alanine (from pyruvate) 2. Aspartate, asparagine (from oxaloacetate) 3. Cysteine (from serine and methionine) 4. Glutamate, glutamine (from α-ketoglutarate) 5. Glycine (from serine) 6. Proline (from glutamate) 7. Serine (from 3-phosphoglycerate) 8. Tyrosine (from phenylalanine) E. Nonessential and not required for protein synthesis 1. Hydroxyproline (made postranslationally from proline) 2. Hydroxylysine (made postranslationally from lysine) III. Acidic, basic, polar, and hydrophobic amino acids A. Acidic amino acids: amino acids that can donate a hydrogen ion (proton) and thereby decrease pH in an aqueous solution 1. -

The Role of Glutamine in the Complex Interaction Between Gut Microbiota and Health: a Narrative Review
International Journal of Molecular Sciences Review The Role of Glutamine in the Complex Interaction between Gut Microbiota and Health: A Narrative Review 1, , 1 1, 1 Simone Perna * y , Tariq A. Alalwan , Zahraa Alaali y , Tahera Alnashaba , Clara Gasparri 2, Vittoria Infantino 3 , Layla Hammad 1, Antonella Riva 4, Giovanna Petrangolini 4 , Pietro Allegrini 4 and Mariangela Rondanelli 5,6 1 Department of Biology, College of Science, University of Bahrain, 32038 Sakhir, Bahrain; [email protected] (T.A.A.); [email protected] (Z.A.); [email protected] (T.A.); [email protected] (L.H.) 2 Endocrinology and Nutrition Unit, Azienda di Servizi alla Persona “Istituto Santa Margherita”, University of Pavia, Pavia 27100, Italy; [email protected] 3 Department of Biomedical Science and Human Oncology, University of Bari, Bari 70121, Italy; [email protected] 4 Research and Development Department, Indena SpA, 20139 Milan, Italy; [email protected] (A.R.); [email protected] (G.P.); [email protected] (P.A.) 5 IRCCS Mondino Foundation, Pavia 27100, Italy; [email protected] 6 Department of Public Health, Experimental and Forensic Medicine, Unit of Human and Clinical Nutrition, University of Pavia, Pavia 27100, Italy * Correspondence: [email protected] or [email protected]; Tel.: +973-39-37-99-46 The two authors contributed equally to this work. y Received: 21 August 2019; Accepted: 20 October 2019; Published: 22 October 2019 Abstract: The scientific literature has demonstrated that glutamine is one of the main beneficial amino acids. It plays an important role in gut microbiota and immunity. This paper provides a critical overview of experimental studies (in vitro, in vivo, and clinical) investigating the efficacy of glutamine and its effect on gut microbiota. -

Effects of Amanita Muscaria Extract on Different in Vitro Neurotoxicity
Food and Chemical Toxicology 132 (2019) 110687 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Effects of Amanita muscaria extract on different in vitro neurotoxicity models at sub-cellular and cellular levels T Magdalena Kondeva-Burdinaa,*, Maria Voynovaa, Aleksandar Shkondrovb, Denitsa Aluania, Virginia Tzankovaa, Ilina Krastevab a Laboratory of Drug Metabolism and Drug Toxicity, Department of Pharmacology, Pharmacotherapy and Toxicology, Faculty of Pharmacy, Medical University of Sofia, 2 Dunav St., 1000, Sofia, Bulgaria b Department of Pharmacognosy, Faculty of Pharmacy, Medical University of Sofia, 2 Dunav St., 1000, Sofia, Bulgaria ARTICLE INFO ABSTRACT Keywords: Muscimol is the main compound found in Amanita muscaria. Several studies have proven that muscimol has Amanita muscaria suppressive effects on essential tremor, without impairing speech and coordination. The effects of muscimol in Isolated rat brain microsomes Parkinson-affected patients is also described in a number of studies. These studies describe the free radical Synaptosomes scavenging and antioxidant activity of the mushroom extract. We have evaluated the possible neuroprotective Mitochondria effects of a standardized extract from A. muscaria, containing high amounts of muscimol, on different models of Neuroblastoma cell line SH-SY5Y neurotoxicity in rat brain microsomes, mitochondria, synaptosomes as well as on neuroblastoma cell line SH- Neuroprotection ff Human recombinant monoaminoxidase-B SY5Y. The possible inhibitory e ect on human recombinant monoaminoxidase-B (hMAOB) enzyme was also enzyme studied. The extract revealed statistically significant neuroprotective effects on the in vitro neurotoxicity models and no inhibitory activity on hMAOB. 1. Introduction without influencing the speech and movements of the patients (Ferreira et al., 2007). -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -

Nucleotide Base Coding and Am1ino Acid Replacemients in Proteins* by Emil L
VOL. 48, 1962 BIOCHEMISTRY: E. L. SAIITH 677 18 Britten, R. J., and R. B. Roberts, Science, 131, 32 (1960). '9 Crestfield, A. M., K. C. Smith, and F. WV. Allen, J. Biol. Chem., 216, 185 (1955). 20 Gamow, G., Nature, 173, 318 (1954). 21 Brenner, S., these PROCEEDINGS, 43, 687 (1957). 22 Nirenberg, M. WV., J. H. Matthaei, and 0. WV. Jones, unpublished data. 23 Crick, F. H. C., L. Barnett, S. Brenner, and R. J. Watts-Tobin, Nature, 192, 1227 (1961). 24 Levene, P. A., and R. S. Tipson, J. Biol. Ch-nn., 111, 313 (1935). 25 Gierer, A., and K. W. Mundry, Nature, 182, 1437 (1958). 2' Tsugita, A., and H. Fraenkel-Conrat, J. Mllot. Biol., in press. 27 Tsugita, A., and H. Fraenkel-Conrat, personal communication. 28 Wittmann, H. G., Naturwissenschaften, 48, 729 (1961). 29 Freese, E., in Structure and Function of Genetic Elements, Brookhaven Symposia in Biology, no. 12 (1959), p. 63. NUCLEOTIDE BASE CODING AND AM1INO ACID REPLACEMIENTS IN PROTEINS* BY EMIL L. SMITHt LABORATORY FOR STUDY OF HEREDITARY AND METABOLIC DISORDERS AND THE DEPARTMENTS OF BIOLOGICAL CHEMISTRY AND MEDICINE, UNIVERSITY OF UTAH COLLEGE OF MEDICINE Communicated by Severo Ochoa, February 14, 1962 The problem of which bases of messenger or template RNA' specify the coding of amino acids in proteins has been largely elucidated by the use of synthetic polyri- bonucleotides.2-7 For these triplet nucleotide compositions (Table 1), it is of in- terest to examine some of the presently known cases of amino acid substitutions in polypeptides or proteins of known structure. -
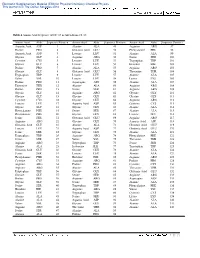
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine