Supplement Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Rearrangements in the Camphor Series : the Decomposition Products of the Methyl Ester of Isoaminocamphonanic Acid
MOLECULAR REARRANGEMENTS IN THE CAMPHOR SERIES. THE DECOMPOSITION PRODUCTS OF THE METHYL ESTER OF ISOAMINOCAMPHONANIC ACID. BY GLENN SEYMOUR SKINNER A. B. Kansas State Manual Training Normal, 1913 A. M. University of Illinois, 1915 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN CHEMISTRY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1917 Digitized by the Internet Archive in 2013 http://archive.org/details/molecularrearranOOskin SK3 UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL .191 7 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPER- VISION BY .QleiirL...S.eymo.nr.....S.]s;i.nner ENTITLED .MQlficulax....E.earrii.ng_meiits in... the .G.amj2hgr....Se^^^^ The I)ejG.QiQ.p.Q.ai.t.i.o.rL...I!r.Q.d.iL.c.t.s Q±....tha.JvIe.l]i3fl....B.st.e..r o.t.....Is..oanDn.ocam^^^^^ Acid. BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF.. .J).Q.C..1i.QX._..Q±....PJlilQ.S..Q.p.llZ. In ChargeCI of Thesis Head of Department Recommendation concurred in :* .<:. TSr:^. Committee on Final Examination* *Required for doctor's degree but not for master's. •^L^n .X o o TABLE OF GONTEIITS. PART I. HISTORICAL. The Camphors ------------------------- 1 The Camphoric Acids --------------------- 2 The Methyl Esters of d-Camphoric and 1-Isocaraphoric Acid - - - 3 The Methyl Esters of d-Camphoramidic and l-Isocamphoramidic Acids 4 The d-Oamphoramidic and 1- Is o camphoramidic Acids ------- 6 The Amino Acids Which are Derived from the d-Gamphoramidic and 1-Isocamphoramidic Acids ---------- 7 The Methyl Esters of the Amino Acid-S - -- -- -- -- -- -- 8 The Decomposition Products of Amino Camphonanic Acid ----- 8 The Decomposition Products of Dihydroaminocampholytic Acid - - 10 The Decomposition of Isoaminocamphonanic Acid ----- - 15 The Decomposition of Isodihydr oaminocampholytic Acid ----- 15 The Unsaturated Acids- -------------------- 1^ The Hydroxy Acids- ---------------------- 1^ The Hydrocarbons ----------------------- 22 The Lactones ------------------------- PART II. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Tio2 DARK ACTIVATION and MICROBIAL STIMULATION
DISSERTATION CATALYTIC STRATEGIES FOR ENHANCING ELECTROCHEMICAL OXIDATION OF 1,4-DIOXANE: TiO2 DARK ACTIVATION AND MICROBIAL STIMULATION Submitted by Jeramy R. Jasmann Department of Chemistry In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Summer 2016 Doctoral Committee: Advisor: Thomas Borch Co-Advisor: Jens Blotevogel Delphine Farmer William Sanford James Neilson Michael Elliot Copyright by Jeramy Roland Jasmann 2016 All Rights Reserved ABSTRACT CATALYTIC STRATEGIES FOR ENHANCING ELECTROCHEMICAL OXIDATION OF 1,4-DIOXANE: TiO2 DARK ACTIVATION AND MICROBIAL STIMULATION 1,4-dioxane, a probable human carcinogen, is an emerging contaminant currently being reviewed by the U.S. Environmental Protection Agency for possible health-based maximum contaminant level regulations. As both stabilizer in commonly used chlorinated solvents and as a widely used solvent in the production of many pharmaceuticals, personal care products (PPCPs), 1,4-dioxane has been detected in surface water, groundwater and wastewater around the U.S. It is resistant to many of the traditional water treatment technologies such as sorption to activated carbon, air stripping, filtering and anaerobic biodegradation making 1,4-dioxane removal difficult and/or expensive. State-of-the art technologies for the removal of 1,4-dioxane usually apply advanced oxidation processes (AOPs) using strong oxidants in combination with UV-light and sometimes titanium dioxide (TiO2) catalyzed photolysis. These approaches require the use of expensive chemical reagents and are limited to ex situ (i.e. pump and treat) applications. Here, at Colorado State University’s Center for Contaminant Hydrology, innovative flow-through electrolytic reactors have been developed for treating groundwater contaminated with organic pollutants. -

Aerosol Fragmentation Driven by Coupling of Acid–Base and Free
Lawrence Berkeley National Laboratory Recent Work Title Aerosol Fragmentation Driven by Coupling of Acid-Base and Free-Radical Chemistry in the Heterogeneous Oxidation of Aqueous Citric Acid by OH Radicals. Permalink https://escholarship.org/uc/item/2hx5q6wq Journal The journal of physical chemistry. A, 121(31) ISSN 1089-5639 Authors Liu, Matthew J Wiegel, Aaron A Wilson, Kevin R et al. Publication Date 2017-08-01 DOI 10.1021/acs.jpca.7b04892 Peer reviewed eScholarship.org Powered by the California Digital Library University of California 1 Aerosol Fragmentation Driven by Coupling of Acid-Base and Free Radical 2 Chemistry in the Heterogeneous Oxidation of Aqueous Citric Acid by OH 3 Radicals 4 Matthew J. Liu,a,b Aaron A. Wiegel,a Kevin R. Wilson,†a and Frances A. Houle†a 5 aLawrence Berkeley National Laboratory, Chemical Sciences Division, Berkeley, CA, USA 6 94702 7 bUniversity of California, Berkeley, Department of Chemical and Biomolecular Engineering, 8 Berkeley, CA, USA 94720 9† Authors to whom correspondence should be addressed: 10Frances A. Houle ([email protected]) and Kevin R. Wilson ([email protected]) 11 12Abstract 13A key uncertainty in the heterogeneous oxidation of carboxylic acids by hydroxyl radicals (OH) 14in aqueous phase aerosol is how the free radical reaction pathways might be altered by acid-base 15chemistry. In particular, if acid-base reactions occur concurrently with acyloxy radical formation 16and unimolecular decomposition of alkoxy radicals, there is a possibility that differences in 17reaction pathways impact the partitioning of organic carbon between the gas and aqueous phases. 18To examine these questions, a kinetic model is developed for the OH initiated oxidation of citric 19acid aerosol at high relative humidity. -

Reactivity of Zinc Halide Complexes Containing Camphor-Derived Guanidine Ligands with Technical Rac-Lactide
inorganics Article Reactivity of Zinc Halide Complexes Containing Camphor-Derived Guanidine Ligands with Technical rac-Lactide Angela Metz 1, Joshua Heck 1, Clara Marie Gohlke 1, Konstantin Kröckert 1, Yannik Louven 1, Paul McKeown 2, Alexander Hoffmann 1, Matthew D. Jones 2 and Sonja Herres-Pawlis 1,* ID 1 Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany; [email protected] (A.M.); [email protected] (J.H.); [email protected] (C.M.G.); [email protected] (K.K.); [email protected] (Y.L.); [email protected] (A.H.) 2 Centre for Sustainable Chemical Technologies, Department of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK; [email protected] (P.M.); [email protected] (M.D.J.) * Correspondence: [email protected]; Tel.: +49-241-80-939-02 Received: 27 October 2017; Accepted: 23 November 2017; Published: 30 November 2017 Abstract: Three new zinc complexes with monoamine–guanidine hybridligands have been prepared, characterized by X-ray crystallography and NMR spectroscopy, and tested in the solvent-free ring-opening polymerization of rac-lactide. Initially the ligands were synthesized from camphoric acid to obtain TMGca and DMEGca and then reacted with zinc(II) halides to form zinc complexes. All complexes have a distorted tetrahedral coordination. They were utilized as catalysts in the solvent-free polymerization of technical rac-lactide at 150 ◦C. Colorless polylactide (PLA) can be produced and after 2 h conversion up to 60% was reached. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide FIRST EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

A Comparison Op Phenylhydrazine Oxalate with Mesoxalic Acid Phenyleydrazone
I724 W. I,. EVANS, W. L. MONG AND F. L. SINKS. [CONTRIEUTXON FROM THE CHEMICAL LABORATORYOB THE OHIO STATE UNIVERSITY.] A COMPARISON OP PHENYLHYDRAZINE OXALATE WITH MESOXALIC ACID PHENYLEYDRAZONE. BY W. L. EVANS,W. I,. MONCAND F. L. SINKS. Received May 26, 1917. While making an examination of the products obtained by the oxidation of potassium lactate by means of potassium permanganate in the presence of small quantities of potassium hydroxide, Paul A. Davis and one of us found a white, leafy, mother-of-pearl, crystalline mass after acidifying the filtrate from the oxide of manganese with acetic acid and then adding a solution of phenylhydrazine in diluted acetic acid.l This substance was associated with the pyruvic acid phenylhydrazone from which it could be easily separated, owing to the fact that it was difficultly soluble in alcohol. This compound was found to be identical in every respect with the compound described by Causse as mesoxalic acid phenylhydra- zone, m. p. 163' to 164'.~ Causse obtained this compound from bismuth mesoxalate which he had prepared by the oxidation of glycerol by means of a mixture of bismuth nitrate, potassium nitrate and nitric acid. On the other hand, Fischer3 describes mesoxalic acid phenylhydrazone as a substance which crystallizes from hot water or dilute alcohol in fine yellow needles. According to Elbers4 it has the following composition: CsH6.NH.N = C(COOH)2, and melts between 163' and 164'. Clemms gives the melting point of this compound as 174' if heated quickly. A comparison of the compound obtained by Fischer and that obtained by the repetition of Causse's experiments, showed that they were two entirely different substances. -

Carbon Dioxide Production in the Oxidation of Organic Acids by Cerium(IV) Under Aerobic and Anaerobic Conditions
JCK(Wiley) RIGHT BATCH Carbon Dioxide Production in the Oxidation of Organic Acids by Cerium(IV) under Aerobic and Anaerobic Conditions KARA BUTLER, OLIVER STEINBOCK,* BETTINA STEINBOCK,* NAR S. DALAL Florida State University, Department of Chemistry, Tallahassee, Florida 32306-3006 Received 16 March 1998; accepted 2 June 1998 ABSTRACT: The stoichiometry of CO2 production during the ceric oxidation of various organic acids is measured under conditions with organic acid excess. Measurements utilize a photo- metric methodology. For anaerobic conditions stoichiometries [CO2]produced : [Ce(IV)]reduced of about 0 (malonic acid), 0.5 (e.g., glyoxylic acid), and 1.0 (oxalic acid) are found. Oxalic acid showed an oxygen-induced decrease of CO2 production, while other compounds such as ma- lonic acid increased the amount of produced CO2 or showed no changes (e.g., tartronic acid). In the case of mesoxalic acid the stoichiometry is increased from about 0.5 to 2.0 due to the presence of molecular oxygen. The results are discussed on the basis of simple reaction mech- anisms demonstrating that useful information on reaction pathways and intermediates can be extracted from these simple measurements. ᭧ 1998 John Wiley & Sons, Inc. Int J Chem Kinet 30: 899±902, 1998 The study of oxidation of relatively low molecular and involve numerous organic acids and radicals as weight carbonic acids by metal ions has been an active intermediates [4,7]. A common characteristic, how- area of kinetics [1]. In particular the oxidation of ma- ever, is that most organic acids are oxidized to carbon lonic acid (CH2(COOH)2) and similar compounds by dioxide. -

List of Chemical Resistance of Materials in Contact with Liquid ANALYSETTE 22 Next & ANALYSETTE 28 Imagesizer LASER PARTICLE SIZER & PARTICLE SIZER
List of chemical resistance of materials in contact with liquid ANALYSETTE 22 NeXT & ANALYSETTE 28 ImageSizer LASER PARTICLE SIZER & PARTICLE SIZER This chart information is based on FRITSCH's knowledge and experience and are intended as a guide and not as a guarantee. We cannot assure the perfect performance of the materials as that depends on many factors as working pressure, pressure picks, fluid, ambient temperature, fluid temperature, concentra‐ tion, duration of exposure, etc… Fritsch GmbH Milling and Sizing Industriestrasse 8 D - 55743 Idar-Oberstein Telephone: +49 (0) 6784/ 70-0 Email: [email protected] Internet: www.fritsch.de Version 04/2020 Index 001 Table of contents Table of contents 1 Materials in contact with liquid.................................................... 4 2 Abbreviations................................................................................. 5 3 List of chemical resistance............................................................. 7 3.1 KEY......................................................................................... 7 3.2 Note....................................................................................... 7 3.3 Table 1.................................................................................... 7 3.4 Table 2.................................................................................. 97 3.5 Table 3................................................................................ 105 - 3 - Materials in contact with liquid 1 Materials in contact with liquid Wet dispersion -
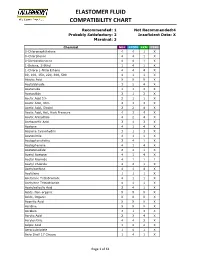
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

Catalytic Oxidation of Glycerol to Dihydroxyacetone
CATALYTIC OXIDATION OF GLYCEROL TO DIHYDROXYACETONE Wenbin Hu, Brian Lowry and Arvind Varma * School of Chemical Engineering, Purdue University, West Lafayette, IN 47907, USA Summary Selective oxidation of glycerol to dihydroxyacetone was investigated using Pt-Bi/C bi-metallic catalyst. The catalysts with different metal loadings, supports and synthesis methods were synthesized and tested in a semi-batch reactor. The optimum catalyst was 3wt%Pt, with sequential 0.6wt%Bi impregnation on Norit Darco 20-40 mesh activated carbon, followed by NaBH 4 reduction, for reaction rate and DHA selectivity. With the optimum catalyst, the reaction conditions (temperature, pressure, pH, etc) in the semi-batch reactor were optimized, with which the maximum DHA yield of 46% was obtained at nearly full glycerol conversion. The catalysts were characterized using BET, ICP-OES, TEM, XRD and XPS techniques. Keywords Glycerol; Dihydroxyacetone; Selective Oxidation; Platinum-Bismuth catalyst Introduction Identifying new uses of glycerol, a waste of biodiesel Experimental production from transesterification of vegetable oils, is of Catalyst Synthesis great current interest. Towards this end, many value-added Pt-Bi/C catalysts with different loadings, supports, chemicals, such as propanediol, propylene, impregnation sequences and reduction methods were epichlorohydrin and methanol, have been explored by synthesized. A typical synthesis procedure is described as chemical companies. The use of glycerol to synthesize follows: activated carbon (AC) was submerged in water these chemicals, however, is less attractive due to the with sonication. A predetermined amount of chloroplatinic limited amount of value added. We use glycerol as a acid hydrate was dissolved in 1.2N HCl and added feedstock for its selective oxidation to produce high-value dropwise to the well-stirred AC slurry. -

Conversion of Glycerol to Lactic Acid Under Low Corrosive Conditions with Homogeneous and Heterogeneous Catalysts
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2011 Conversion of Glycerol to Lactic Acid under Low Corrosive Conditions with Homogeneous and Heterogeneous Catalysts Lu Chen [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Bioresource and Agricultural Engineering Commons Recommended Citation Chen, Lu, "Conversion of Glycerol to Lactic Acid under Low Corrosive Conditions with Homogeneous and Heterogeneous Catalysts. " Master's Thesis, University of Tennessee, 2011. https://trace.tennessee.edu/utk_gradthes/960 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Lu Chen entitled "Conversion of Glycerol to Lactic Acid under Low Corrosive Conditions with Homogeneous and Heterogeneous Catalysts." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Biosystems Engineering. Xiaofei P. Ye, Major Professor We have read this thesis and recommend its acceptance: Douglas G. Hayes, Qixin Zhong Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Conversion of Glycerol to Lactic Acid under Low Corrosive Conditions with Homogeneous and Heterogeneous Catalysts A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Lu Chen August 2011 Copyright © 2011 by Lu Chen All rights reserved.