William Albert Noyes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

William Albert Noyes
NATIONAL ACADEMY OF SCIENCES WILLIAM ALBERT NOYES 1857—1941 A Biographical Memoir by ROGER ADAMS Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1952 NATIONAL ACADEMY OF SCIENCES WASHINGTON D.C. WILLIAM ALBERT NOYES1 BY ROGER ADAMS William Albert Noyes was born November 6, 1857 on a farm near Independence, Iowa. He was the seventh generation of the Noyes family in America, being descended from Nicholas Noyes who came with his elder brother, James, from Choulder- ton, Wiltshire, in 1633. Their father had been a clergyman but had died before the sons left England. The two brothers settled first in Medford, Massachusetts but in 1655 moved to Newbury. Samuel, of the third generation in this direct line, moved to Abington about 1713 and there he and the succeeding generations lived for over one hundred and forty years. Spencer W. Noyes, the great grandson of Samuel, was ad- mitted as a student to Phillips Academy at Andover, Massachu- setts, March 21, 1837. He married Mary Packard-related to the famous family of shoe manufacturers-and they had three children when they left: Abington and migrated to Iowa in 185j. When the father with his family went west to open up a home- stead, he found conditions typical of the prairies and life was necessarily primitive. In the east he had been a cobbler by trade so had had little experience working the land. The early years were difficult ones because of the hard work in establishing a farm home, getting land into cultivation, raising food and storing sufficient for winter needs. -

Molecular Rearrangements in the Camphor Series : the Decomposition Products of the Methyl Ester of Isoaminocamphonanic Acid
MOLECULAR REARRANGEMENTS IN THE CAMPHOR SERIES. THE DECOMPOSITION PRODUCTS OF THE METHYL ESTER OF ISOAMINOCAMPHONANIC ACID. BY GLENN SEYMOUR SKINNER A. B. Kansas State Manual Training Normal, 1913 A. M. University of Illinois, 1915 THESIS Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN CHEMISTRY IN THE GRADUATE SCHOOL OF THE UNIVERSITY OF ILLINOIS 1917 Digitized by the Internet Archive in 2013 http://archive.org/details/molecularrearranOOskin SK3 UNIVERSITY OF ILLINOIS THE GRADUATE SCHOOL .191 7 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPER- VISION BY .QleiirL...S.eymo.nr.....S.]s;i.nner ENTITLED .MQlficulax....E.earrii.ng_meiits in... the .G.amj2hgr....Se^^^^ The I)ejG.QiQ.p.Q.ai.t.i.o.rL...I!r.Q.d.iL.c.t.s Q±....tha.JvIe.l]i3fl....B.st.e..r o.t.....Is..oanDn.ocam^^^^^ Acid. BE ACCEPTED AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF.. .J).Q.C..1i.QX._..Q±....PJlilQ.S..Q.p.llZ. In ChargeCI of Thesis Head of Department Recommendation concurred in :* .<:. TSr:^. Committee on Final Examination* *Required for doctor's degree but not for master's. •^L^n .X o o TABLE OF GONTEIITS. PART I. HISTORICAL. The Camphors ------------------------- 1 The Camphoric Acids --------------------- 2 The Methyl Esters of d-Camphoric and 1-Isocaraphoric Acid - - - 3 The Methyl Esters of d-Camphoramidic and l-Isocamphoramidic Acids 4 The d-Oamphoramidic and 1- Is o camphoramidic Acids ------- 6 The Amino Acids Which are Derived from the d-Gamphoramidic and 1-Isocamphoramidic Acids ---------- 7 The Methyl Esters of the Amino Acid-S - -- -- -- -- -- -- 8 The Decomposition Products of Amino Camphonanic Acid ----- 8 The Decomposition Products of Dihydroaminocampholytic Acid - - 10 The Decomposition of Isoaminocamphonanic Acid ----- - 15 The Decomposition of Isodihydr oaminocampholytic Acid ----- 15 The Unsaturated Acids- -------------------- 1^ The Hydroxy Acids- ---------------------- 1^ The Hydrocarbons ----------------------- 22 The Lactones ------------------------- PART II. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Alpha Chi Sigma Fraternity Sourcebook, 2013-2014 This Sourcebook Is the Property Of
Alpha Chi Sigma Sourcebook A Repository of Fraternity Knowledge for Reference and Education Academic Year 2013-2014 Edition 1 l Alpha Chi Sigma Fraternity Sourcebook, 2013-2014 This Sourcebook is the property of: ___________________________________________________ ___________________________________________________ Full Name Chapter Name ___________________________________________________ Pledge Class ___________________________________________________ ___________________________________________________ Date of Pledge Ceremony Date of Initiation ___________________________________________________ ___________________________________________________ Master Alchemist Vice Master Alchemist ___________________________________________________ ___________________________________________________ Master of Ceremonies Reporter ___________________________________________________ ___________________________________________________ Recorder Treasurer ___________________________________________________ ___________________________________________________ Alumni Secretary Other Officer Members of My Pledge Class ©2013 Alpha Chi Sigma Fraternity 6296 Rucker Road, Suite B | Indianapolis, IN 46220 | (800) ALCHEMY | [email protected] | www.alphachisigma.org Click on the blue underlined terms to link to supplemental content. A printed version of the Sourcebook is available from the National Office. This document may be copied and distributed freely for not-for-profit purposes, in print or electronically, provided it is not edited or altered in any -

THE NINETY-THIRD PRESENTATION of the WILLARD GIBBS MEDAL (Founded by William A
http:/chicagoacs.org MAY• 2004 THE NINETY-THIRD PRESENTATION OF THE WILLARD GIBBS MEDAL (Founded by William A. Converse) to PROFESSOR RONALD BRESLOW sponsored by the CHICAGO SECTION AMERICAN CHEMICAL SOCIETY FRIDAY, MAY 21, 2004 North Shore Lights at The iation for a nametag , and your check. Acceptance of the Award Hotel Moraine Be sure to include your address. 700 North Sheridan Road Tables fo r ten are availab le. If you Highwood, Illinois would like a table for a group, please 847-433-6366 put the ir names on a separate sheet and include it with your registration. DIRECTIONS TO THE MEETING From the North or South: Take 1-294 (continued on page 2) (the TriState Tollway) to Route 22. Exit east, take it to Route 41 (Skokie Hwy). AWARD CEREMONY 8:30 P.M. Turn north to the next exit, Old Elm. Go east on Old Elm to Sheridan Road Oust The Willard Gibbs Medal across some railroad tracks) . Turn right/south for 3/4 mile. The hotel is on Milt Levenberg, Chair the right. Chicago Section, ACS From Downtown: Take the Kennedy Introduction of the Medalist Expressway north. At the split , follow the Edens Expressway , which turns Madeleine Jacobs Executive Director & CEO, ACS into Skokie Highway past Lake Cook Dr. Ronald Breslow Road. Continue north to Old Elm Road. Presentation of the Medal Samuel Latham Mitchill Professor of Turn right/east on Old Elm and follow Chemistry and University Professor the directions above to the hotel. Dr. Charles P. Casey Department of Chemistry President, ACS Columbia University Parking: Free New York, NY RECEPTION 6:00-7:00 P.M. -

William Hughes Miller Biographical Notes
2490 J. Phys. Chem. A 2001, 105, 2490 William Hughes Miller Biographical Notes Personal: Born: March 16, 1941, Kosciusko, Mississippi Married: Margaret Ann Westbrook, (two daughters; Alison b. 1970, Emily b. 1972) Education and Positions: 1956-59 Provine High School, Jackson, MS, Valedictorian 1959-63 Georgia Institute of Technology, General Motors National Scholarship, B. S. 1963 (Chemistry), Phi Kappa Phi Cup (Valedictorian) 1963-67 Harvard University, National Science Foundation Fellow, A. M. 1964 (Chemistry) Ph.D. 1967 (Chemical Physics), E. Bright Wilson, Jr., Research Director 1967-68 NATO Postdoctoral Fellow, University of Freiburg, Germany 1967-69 Junior Fellow, Society of Fellows, Harvard University 1969-72 Assistant Professor, Department of Chemistry, University of California, Berkeley 1969-present Staff Senior Scientist, Chemical Sciences Division, Lawrence Berkeley National Laboratory 1972-74 Associate Professor, Department of Chemistry, University of California, Berkeley 1974-present Professor, Department of Chemistry, University of California, Berkeley 1984-88 Vice-Chairman, Department of Chemistry, University of California, Berkeley 1989-93 Chairman, Department of Chemistry, University of California, Berkeley 1998-2001 Chancellor’s Research Professor, University of California, Berkeley 1999-present Kenneth S. Pitzer Distinguished Professor of Chemistry, University of California, Berkeley Awards and Honors: Alfred P. Sloan Research Fellow, 1970-1972 Camille and Henry Dreyfus Teacher-Scholar, 1973-1979 Annual Prize of the -

William H. Miller's Curriculum Vitae
Curriculum Vitae WILLIAM HUGHES MILLER Personal: Born: 16 March 1941, Kosciusko, Mississippi, USA Married: Margaret Ann Westbrook, (two daughters, Alison b. 1970, Emily b. 1972) Education and Positions: 1956-59 Provine High School, Jackson, MS, Valedictorian 1959-63 Georgia Institute of Technology, General Motors National Scholarship, B.S. 1963 (Chemistry), Phi Kappa Phi Cup (Valedictorian) 1963-67 Harvard University, National Science Foundation Fellow, A.M. 1964 (Chemistry) Ph.D. 1967 (Chemical Physics), E. Bright Wilson, Jr., Research Director 1967-68 NATO Postdoctoral Fellow, University of Freiburg, Germany 1967-69 Junior Fellow, Society of Fellows, Harvard University 1969-72 Assistant Professor, Department of Chemistry, University of California, Berkeley 1969-present Staff Senior Scientist, Chemical Sciences Division, Lawrence Berkeley National Laboratory 1972-74 Associate Professor, Department of Chemistry, University of California, Berkeley 1974-2010 Professor, Department of Chemistry, University of California, Berkeley 1984-88 Vice-Chairman, Department of Chemistry, University of California, Berkeley 1989-93 Chairman, Department of Chemistry, University of California, Berkeley 1998-2001 Chancellor’s Research Professor, University of California, Berkeley 1999-2012 Kenneth S. Pitzer Distinguished Professor of Chemistry, University of California, Berkeley 2012-present Kenneth S. Pitzer Distinguished Professor Emeritus, and Professor of the Graduate School, University of California, Berkeley 9/13 1 Honors: Alfred P. Sloan Research -

Reactivity of Zinc Halide Complexes Containing Camphor-Derived Guanidine Ligands with Technical Rac-Lactide
inorganics Article Reactivity of Zinc Halide Complexes Containing Camphor-Derived Guanidine Ligands with Technical rac-Lactide Angela Metz 1, Joshua Heck 1, Clara Marie Gohlke 1, Konstantin Kröckert 1, Yannik Louven 1, Paul McKeown 2, Alexander Hoffmann 1, Matthew D. Jones 2 and Sonja Herres-Pawlis 1,* ID 1 Institute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany; [email protected] (A.M.); [email protected] (J.H.); [email protected] (C.M.G.); [email protected] (K.K.); [email protected] (Y.L.); [email protected] (A.H.) 2 Centre for Sustainable Chemical Technologies, Department of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK; [email protected] (P.M.); [email protected] (M.D.J.) * Correspondence: [email protected]; Tel.: +49-241-80-939-02 Received: 27 October 2017; Accepted: 23 November 2017; Published: 30 November 2017 Abstract: Three new zinc complexes with monoamine–guanidine hybridligands have been prepared, characterized by X-ray crystallography and NMR spectroscopy, and tested in the solvent-free ring-opening polymerization of rac-lactide. Initially the ligands were synthesized from camphoric acid to obtain TMGca and DMEGca and then reacted with zinc(II) halides to form zinc complexes. All complexes have a distorted tetrahedral coordination. They were utilized as catalysts in the solvent-free polymerization of technical rac-lactide at 150 ◦C. Colorless polylactide (PLA) can be produced and after 2 h conversion up to 60% was reached. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide FIRST EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Universiv Micrdrilms International 300 N
INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1.The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. For blurred pages, a good image of the page can be found in the adjacent frame. If copyrighted materials were deleted, a target note will appear listing the pages in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photographed, a definite method of “sectioning” the material has been followed. It is customary to begin filming at the upper left hand comer of a large sheet and to continue from left to right in equal sections with small overlaps. If necessary, sectioning is continued again-beginning below the first row and continuing on until complete. -

List of Chemical Resistance of Materials in Contact with Liquid ANALYSETTE 22 Next & ANALYSETTE 28 Imagesizer LASER PARTICLE SIZER & PARTICLE SIZER
List of chemical resistance of materials in contact with liquid ANALYSETTE 22 NeXT & ANALYSETTE 28 ImageSizer LASER PARTICLE SIZER & PARTICLE SIZER This chart information is based on FRITSCH's knowledge and experience and are intended as a guide and not as a guarantee. We cannot assure the perfect performance of the materials as that depends on many factors as working pressure, pressure picks, fluid, ambient temperature, fluid temperature, concentra‐ tion, duration of exposure, etc… Fritsch GmbH Milling and Sizing Industriestrasse 8 D - 55743 Idar-Oberstein Telephone: +49 (0) 6784/ 70-0 Email: [email protected] Internet: www.fritsch.de Version 04/2020 Index 001 Table of contents Table of contents 1 Materials in contact with liquid.................................................... 4 2 Abbreviations................................................................................. 5 3 List of chemical resistance............................................................. 7 3.1 KEY......................................................................................... 7 3.2 Note....................................................................................... 7 3.3 Table 1.................................................................................... 7 3.4 Table 2.................................................................................. 97 3.5 Table 3................................................................................ 105 - 3 - Materials in contact with liquid 1 Materials in contact with liquid Wet dispersion -
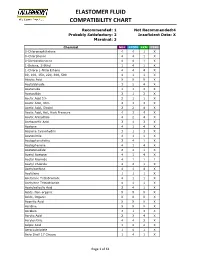
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air,