Microvertebrates from the Basal Rhaetian Bone Bed (Latest Triassic) At
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Late Permian Ichthyofauna from the Zechstein Basin, Lithuania-Latvia Region
bioRxiv preprint doi: https://doi.org/10.1101/554998; this version posted February 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 A late Permian ichthyofauna from the Zechstein Basin, Lithuania-Latvia Region 2 3 Darja Dankina-Beyer1*, Andrej Spiridonov1,4, Ģirts Stinkulis2, Esther Manzanares3, 4 Sigitas Radzevičius1 5 6 1 Department of Geology and Mineralogy, Vilnius University, Vilnius, Lithuania 7 2 Chairman of Bedrock Geology, Faculty of Geography and Earth Sciences, University 8 of Latvia, Riga, Latvia 9 3 Department of Botany and Geology, University of Valencia, Valencia, Spain 10 4 Laboratory of Bedrock Geology, Nature Research Centre, Vilnius, Lithuania 11 12 *[email protected] (DD-B) 13 14 Abstract 15 The late Permian is a transformative time, which ended in one of the most 16 significant extinction events in Earth’s history. Fish assemblages are a major 17 component of marine foods webs. The macroevolution and biogeographic patterns of 18 late Permian fish are currently insufficiently known. In this contribution, the late Permian 19 fish fauna from Kūmas quarry (southern Latvia) is described for the first time. As a 20 result, the studied late Permian Latvian assemblage consisted of isolated 21 chondrichthyan teeth of Helodus sp., ?Acrodus sp., ?Omanoselache sp. and 22 euselachian type dermal denticles as well as many osteichthyan scales of the 23 Haplolepidae and Elonichthydae; numerous teeth of Palaeoniscus, rare teeth findings of 1 bioRxiv preprint doi: https://doi.org/10.1101/554998; this version posted February 20, 2019. -

Revision Des Faunes De Vertebres Du Site De Proven Cheres-Sur-Meuse (Trias Terminal, Nord-Est De La France)
REVISION DES FAUNES DE VERTEBRES DU SITE DE PROVEN CHERES-SUR-MEUSE (TRIAS TERMINAL, NORD-EST DE LA FRANCE) par Gilles CUNY * SOMMAIRE Page Résumé, Abstract ..................... : . .. 103 Introduction ..................................................................... 103 Historique. 103 Révision des anciennes collections ................................................... 105 Muséum National d'Histoire Naturelle ................... 105 Université Pierre et Marie Curie. .. .. .. 106 Besançon . 120 Dijon. .. .. .. .. 121 Langres - Saint-Dizier. 121 Lyon ....................................................................... 123 Etude du matériel récent. 126 Discussion ...................................................................... 127 Conclusion ................................................................. :. 129 Remerciements. .. 130 Bibliographie . .. 130 Légendes des planches. 134 * Laboratoire de Paléontologie des Vertébrés, Université Pierre et Marie Curie - Boîte 106, 4 place Jussieu, 75252 PARIS cédex OS, France. Mots-clés: Trias, Rhétien, Poissons, Amphibiens, Reptiles Key-words: Triassic, Rhetian, Fishes, Amphibians, Reptiles PalaeoveT/ebra/a. Montpellier, 24 (1-2): 10H34. 6 fig .• 3 pl. (Reçu le 23 Février 1994. accepté le 15 Mai 1994. publié le 14 Juin 1995) -~----------------- - --.------- --------------- 500MHRES -- ------ - ------ -- --- ---- -------- ---.- A--- ..L -L ~ ~ 1.10 1.00 1 <6~::<:<=.: :~:~:::,'EE: J '_ 0.50 =...- ~ ::_-_____ -___ ---___ ~~:: ~-- __ .- _______ ~:: ----___ ~: ~-____ : ~ ~-. ~ ~ '_____ -

The Early Triassic Jurong Fish Fauna, South China Age, Anatomy, Taphonomy, and Global Correlation
Global and Planetary Change 180 (2019) 33–50 Contents lists available at ScienceDirect Global and Planetary Change journal homepage: www.elsevier.com/locate/gloplacha Research article The Early Triassic Jurong fish fauna, South China: Age, anatomy, T taphonomy, and global correlation ⁎ Xincheng Qiua, Yaling Xua, Zhong-Qiang Chena, , Michael J. Bentonb, Wen Wenc, Yuangeng Huanga, Siqi Wua a State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences (Wuhan), Wuhan 430074, China b School of Earth Sciences, University of Bristol, BS8 1QU, UK c Chengdu Center of China Geological Survey, Chengdu 610081, China ARTICLE INFO ABSTRACT Keywords: As the higher trophic guilds in marine food chains, top predators such as larger fishes and reptiles are important Lower Triassic indicators that a marine ecosystem has recovered following a crisis. Early Triassic marine fishes and reptiles Fish nodule therefore are key proxies in reconstructing the ecosystem recovery process after the end-Permian mass extinc- Redox condition tion. In South China, the Early Triassic Jurong fish fauna is the earliest marine vertebrate assemblage inthe Ecosystem recovery period. It is constrained as mid-late Smithian in age based on both conodont biostratigraphy and carbon Taphonomy isotopic correlations. The Jurong fishes are all preserved in calcareous nodules embedded in black shaleofthe Lower Triassic Lower Qinglong Formation, and the fauna comprises at least three genera of Paraseminotidae and Perleididae. The phosphatic fish bodies often show exceptionally preserved interior structures, including net- work structures of possible organ walls and cartilages. Microanalysis reveals the well-preserved micro-structures (i.e. collagen layers) of teleost scales and fish fins. -

American Museum Published by the American Museum of Natural History Central Park West at 79Th Street New York, N.Y
NovitatesAMERICAN MUSEUM PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET NEW YORK, N.Y. 10024 U.S.A. NUMBER 2718 NOVEMBER 19, 1981 JOHN G. MAISEY Studies on the Paleozoic Selachian Genus Ctenacanthus Agassiz No. 1. Historical Review and Revised Diagnosis of Ctenacanthus, With a List of Referred Taxa AMERICAN MUSEUM Novitates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N.Y. 10024 Number 2718, pp. 1-22, figs. 1-21 November 19, 1981 Studies on the Paleozoic Selachian Genus Ctenacanthus Agassiz No. 1. Historical Review and Revised Diagnosis of Ctenacanthus, With a List of Referred Taxa JOHN G. MAISEY1 ABSTRACT Ctenacanthus Agassiz is a genus of elasmo- elasmobranch finspines, whereas others resemble branch, originally recognized by its dorsal fin- hybodontid finspines. The fish described by Dean spines but now known from more complete re- as Ctenacanthus clarkii should be referred to C. mains. However, many other fossils, including compressus. Both C. clarkii and C. compressus isolated spines and complete fish, have been in- finspines are sufficiently like those of C. major cluded in Ctenacanthus, although the spines dif- for these species to remain within the genus. fer from those of the type species, C. major, and Ctenacanthus compressus is the only articulated from other presumably related species. Earlier Paleozoic shark so far described which can be diagnoses of Ctenacanthus are critically reviewed assigned to Ctenacanthus. Ctenacanthus costel- and the significance of previous diagnostic latus finspines are not like those of C. major, but changes is discussed. -

Introduction
BELGIAN GEOLOGICAL SURVEY. Professional Paper, 278: Elasmobranches et Stratigraphie (1994), 11-21,1995. Chondrichthyens mésozoïques du Grand Duché de Luxembourg par Dominique DELSATE (*) Résumé: Rappel des faunes de Chondrichthyens du Grand Duché de Luxembourg précédemment décrites. Des spécimens nouveaux sont signalés. Description d'une dent d 'Acrodus de l'Hettangien de Burmerange, et d'une dent â'Hybodus grossiconus du Toarcien inférieur de Soleuvre. Deux aiguillons de nageoire dorsale d'Hybodontoidea, de l'Hettangien (Brouch) et du Toarcien (Soleuvre), sont présentés. Identification formelle du genre Sphenodus parmi les dents du Bajocien luxembourgeois. Mots-clés: Chondrichthyes, Mésozoïque, Luxembourg. Abstract: Previously described Chondrichthyes teeth from the Grand Duchy of Luxembourg are reminded. New specimens are reported. A tooth of Acrodus from the Hettangian of Burmerange and a tooth of Hybodus grossiconus from the lower Toarcian of Soleuvre are described. A very large fin spine from the Lower Toarcian of Soleuvre and a small one from the Hettangian of Brouch are introduced. Some Bajocian teeth are assigned to the genus Sphenodus. Key words: Chondrichthyes, Mesozoic, Luxembourg Kurzfassung: Ein Zahn von Acrodus aus dem Hettangium von Biirmeningen wird beschrieben; ein Zahn aus dem Unter-Toarcium von Zolver wird Hybodus grossiconus zugeordnet. Zwei fossile Stacheln von Rückenflossen, einer aus dem Hettangium (Brouch) und einer aus dem Toarcium (Soleuvre) stammen von Hybodontoidea. Einige Zähne des Toarciums und Bajociums werden der Gattung Sphenodus zugewiesen. Schlüsselwörter: Chondrichthyes, Mesozoicum, Luxemburg * Dominique DELSATE: Centre de Recherches Lorraines (B-6760 Ethe) ou 5 Rue du Quartier (B-6792 Battincourt), Belgique. Introduction Le Centre de Recherches Lorraines, le Service Géologique de Belgique et l'Institut Royal des Sciences Naturelles de Belgique ont entrepris conjointement l'exploration systématique des différents niveaux du Mésozoïque grand- ducal ainsi que l'examen des collections nationales ou privées existantes. -

Copyrighted Material
06_250317 part1-3.qxd 12/13/05 7:32 PM Page 15 Phylum Chordata Chordates are placed in the superphylum Deuterostomia. The possible rela- tionships of the chordates and deuterostomes to other metazoans are dis- cussed in Halanych (2004). He restricts the taxon of deuterostomes to the chordates and their proposed immediate sister group, a taxon comprising the hemichordates, echinoderms, and the wormlike Xenoturbella. The phylum Chordata has been used by most recent workers to encompass members of the subphyla Urochordata (tunicates or sea-squirts), Cephalochordata (lancelets), and Craniata (fishes, amphibians, reptiles, birds, and mammals). The Cephalochordata and Craniata form a mono- phyletic group (e.g., Cameron et al., 2000; Halanych, 2004). Much disagree- ment exists concerning the interrelationships and classification of the Chordata, and the inclusion of the urochordates as sister to the cephalochor- dates and craniates is not as broadly held as the sister-group relationship of cephalochordates and craniates (Halanych, 2004). Many excitingCOPYRIGHTED fossil finds in recent years MATERIAL reveal what the first fishes may have looked like, and these finds push the fossil record of fishes back into the early Cambrian, far further back than previously known. There is still much difference of opinion on the phylogenetic position of these new Cambrian species, and many new discoveries and changes in early fish systematics may be expected over the next decade. As noted by Halanych (2004), D.-G. (D.) Shu and collaborators have discovered fossil ascidians (e.g., Cheungkongella), cephalochordate-like yunnanozoans (Haikouella and Yunnanozoon), and jaw- less craniates (Myllokunmingia, and its junior synonym Haikouichthys) over the 15 06_250317 part1-3.qxd 12/13/05 7:32 PM Page 16 16 Fishes of the World last few years that push the origins of these three major taxa at least into the Lower Cambrian (approximately 530–540 million years ago). -
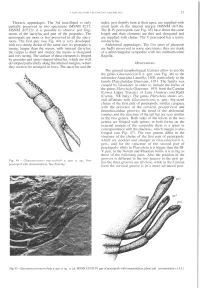
Thoracic Appendages. the 3Rd Maxilliped Is Only Partially
A NEW DECAPOD CRUSTACEAN ASSEMBLAGE 11 Thoracic appendages. The 3rd maxilliped is only index, just slightly bent at their apex, are supplied with partially preserved in two specimens (MSNB 8237; small teeth on the internal margin (MSNM i 10736). MSNM i 10732): it is possible to observe just frag- The II-IV pereiopods (see Fig. 45) have about the same ments of the dactylus and part of the propodus. The length and their elements are thin and elongated and pereiopods are more or less preserved in all the speci- are supplied with chelae. The V pereiopod has a termi- mens. The first pair (see Fig. 44) is very developed, nal dactylus. with two strong chelae of the same size; its propodus is Abdominal appendages. The five pairs of pleopods strong, longer than the merus, with internal dactylus; are badly preserved in some specimens; they are made the carpus is short and stocky; the merus is elongated of a subrectangular sympodite with two multiarticulate and very strong. The surface of these elements is fringed flagella. by granules and spine-shaped tubercles, which are well developed particularly along the internal margins, where Observations they seem to be arranged in rows. The dactylus and the The general morphological features allow to ascribe the genus Glaessnericaris n. gen. (see Fig. 46) to the infraorder Astacidea Latreille, 1803, particularly to the family Platychelidae Glaessner, 1931. The family was created by Glaessner in order to include the forms of the genus Platychela Glaessner, 1931 from the Carnian (Lower Upper Triassic) of Lunz (Austria) and Raibl (Carnia, NE Italy). -

Database of Bibliography of Living/Fossil
www.shark-references.com Version 16.01.2018 Bibliography database of living/fossil sharks, rays and chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) Papers of the year 2017 published by Jürgen Pollerspöck, Benediktinerring 34, 94569 Stephansposching, Germany and Nicolas Straube, Munich, Germany ISSN: 2195-6499 DOI: 10.13140/RG.2.2.32409.72801 copyright by the authors 1 please inform us about missing papers: [email protected] www.shark-references.com Version 16.01.2018 Abstract: This paper contains a collection of 817 citations (no conference abstracts) on topics related to extant and extinct Chondrichthyes (sharks, rays, and chimaeras) as well as a list of Chondrichthyan species and hosted parasites newly described in 2017. The list is the result of regular queries in numerous journals, books and online publications. It provides a complete list of publication citations as well as a database report containing rearranged subsets of the list sorted by the keyword statistics, extant and extinct genera and species descriptions from the years 2000 to 2017, list of descriptions of extinct and extant species from 2017, parasitology, reproduction, distribution, diet, conservation, and taxonomy. The paper is intended to be consulted for information. In addition, we provide data information on the geographic and depth distribution of newly described species, i.e. the type specimens from the years 1990 to 2017 in a hot spot analysis. New in this year's POTY is the subheader "biodiversity" comprising a complete list of all valid chimaeriform, selachian and batoid species, as well as a list of the top 20 most researched chondrichthyan species. Please note that the content of this paper has been compiled to the best of our abilities based on current knowledge and practice, however, possible errors cannot entirely be excluded. -

Micro-Computed Tomography Imaging Reveals the Development of A
www.nature.com/scientificreports OPEN Micro-computed tomography imaging reveals the development of a unique tooth mineralization pattern Received: 20 February 2019 Accepted: 18 June 2019 in mackerel sharks (Chondrichthyes; Published: xx xx xxxx Lamniformes) in deep time Patrick L. Jambura 1, René Kindlimann2, Faviel López-Romero1, Giuseppe Marramà 1, Cathrin Pfaf 1, Sebastian Stumpf 1, Julia Türtscher1, Charlie J. Underwood 3, David J. Ward 4 & Jürgen Kriwet 1 The cartilaginous fshes (Chondrichthyes) have a rich fossil record which consists mostly of isolated teeth and, therefore, phylogenetic relationships of extinct taxa are mainly resolved based on dental characters. One character, the tooth histology, has been examined since the 19th century, but its implications on the phylogeny of Chondrichthyes is still in debate. We used high resolution micro-CT images and tooth sections of 11 recent and seven extinct lamniform sharks to examine the tooth mineralization processes in this group. Our data showed similarities between lamniform sharks and other taxa (a dentinal core of osteodentine instead of a hollow pulp cavity), but also one feature that has not been known from any other elasmobranch fsh: the absence of orthodentine. Our results suggest that this character resembles a synapomorphic condition for lamniform sharks, with the basking shark, Cetorhinus maximus, representing the only exception and reverted to the plesiomorphic tooth histotype. Additionally, †Palaeocarcharias stromeri, whose afliation still is debated, shares the same tooth histology only known from lamniform sharks. This suggests that †Palaeocarcharias stromeri is member of the order Lamniformes, contradicting recent interpretations and thus, dating the origin of this group back at least into the Middle Jurassic. -

Morphometric Discriminant Analysis of Isolated Chondrichthyan Scales for Palaeoecological Inferences: the Middle Triassic Of
Journal of Iberian Geology 40 (1) 2014: 87-97 http://dx.doi.org/10.5209/rev_JIGE.2014.v40.n1.44089 www.ucm.es /info/estratig/journal.htm ISSN (print): 1698-6180. ISSN (online): 1886-7995 Morphometric Discriminant Analysis of isolated chondrichthyan scales for palaeoecological inferences: the Middle Triassic of the Iberian Chain (Spain) as a case of study H. Ferrón1, C. Pla1, C. Martínez-Pérez1,2, M.J. Escudero-Mozo3,4, H. Botella1* 1Departamento de Geología, Universidad de Valencia, Avda. Dr. Moliner, 50, 46100 Burjassot, Spain. 2School of Earth Sciences, University of Bristol, Wills Memorial Building, Queen’s Road, Bristol BS8 1RJ, United Kingdom. 3 Departamento de Estratigrafía, Universidad Complutense de Madrid, C/José Antonio Novais 12, 28040 Madrid, Spain 4 Instituto de Geociencias IGEO (CSIC,UCM), C/José Antonio Novais 12, 28040 Madrid, Spain e-mail addresses: [email protected] (H.F.), [email protected] (C.P.), [email protected] (C.M.-P.); [email protected] (M.J.E.-M.) [email protected] (H.B., *corresponding author) Received: 12 December 2012 / Accepted: 4 December 2013 / Available online: 25 February 2014 Abstract Palaeontological studies on exosqueletal disarticulated remains of chondrichthyans have focused on teeth and only less interest has been paid to scales due their limited taxonomic and systematic significance. However, classical works linking the morphology and the function of the squamation in extant sharks suggest that, despite their limited taxonomic value, the study of isolated scales can be a useful tool for palaeoenvironmental and palaeoecological inferences. Following this idea, we have analyzed the fossil record of shark scales from two Middle Triassic sections of the Iberian Chain (Spain), identifying different functional types by means of a morphometric discriminant analysis. -

Fossils Provide Better Estimates of Ancestral Body Size Than Do Extant
Acta Zoologica (Stockholm) 90 (Suppl. 1): 357–384 (January 2009) doi: 10.1111/j.1463-6395.2008.00364.x FossilsBlackwell Publishing Ltd provide better estimates of ancestral body size than do extant taxa in fishes James S. Albert,1 Derek M. Johnson1 and Jason H. Knouft2 Abstract 1Department of Biology, University of Albert, J.S., Johnson, D.M. and Knouft, J.H. 2009. Fossils provide better Louisiana at Lafayette, Lafayette, LA estimates of ancestral body size than do extant taxa in fishes. — Acta Zoologica 2 70504-2451, USA; Department of (Stockholm) 90 (Suppl. 1): 357–384 Biology, Saint Louis University, St. Louis, MO, USA The use of fossils in studies of character evolution is an active area of research. Characters from fossils have been viewed as less informative or more subjective Keywords: than comparable information from extant taxa. However, fossils are often the continuous trait evolution, character state only known representatives of many higher taxa, including some of the earliest optimization, morphological diversification, forms, and have been important in determining character polarity and filling vertebrate taphonomy morphological gaps. Here we evaluate the influence of fossils on the interpretation of character evolution by comparing estimates of ancestral body Accepted for publication: 22 July 2008 size in fishes (non-tetrapod craniates) from two large and previously unpublished datasets; a palaeontological dataset representing all principal clades from throughout the Phanerozoic, and a macroecological dataset for all 515 families of living (Recent) fishes. Ancestral size was estimated from phylogenetically based (i.e. parsimony) optimization methods. Ancestral size estimates obtained from analysis of extant fish families are five to eight times larger than estimates using fossil members of the same higher taxa. -

Vertebrate Fauna from the Late Triassic Tiki Formation of India: New Finds and Their Biostratigraphic Implications
The Palaeobotanist 65(2016): 47–59 0031–0174/2016 Vertebrate fauna from the Late Triassic Tiki Formation of India: new finds and their biostratigraphic implications SANGHAMITRA RAY1*, MOHD. SHAFI BHAT1, DEBARATI MUKHERJEE2 AND P.M. DATTA3 1Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur 721302, India. 2Geological Studies Unit, Indian Statistical Institute, 203 B.T. Road, Kolkata 700108, India. 3Greenwood Housing Cooperative Society Ltd., 315B Upen Banerjee Road, Kolkata 700060, India. *Corresponding author: [email protected] (Received 17 August, 2015; revised version accepted 06 February, 2016) ABSTRACT Ray S, Bhat MS, Mukherjee D & Datta PM 2016. Vertebrate fauna from the Late Triassic Tiki Formation of India: new finds and their biostratigraphic implications. The Palaeobotanist 65(1): 47–59. Recent work on the Tiki Formation has resulted in the collection of new and varied vertebrate micro–and megafossils, including a new bonebed containing low diversity, mono–dominant, multitaxic vertebrate accumulation where the rhynchosaur, Hyperodapedon tikiensis constitute the dominant component. This bonebed has also yielded a large traversodontid cynodont Ruberodon roychowdhurii. In addition, there are several diagnostic postcrania such as vertebrae and incomplete limb bones belonging to a basal saurischian dinosaur. Systematic exploration and collection has yielded numerous isolated teeth and postcrania of small vertebrates such as different types of freshwater sharks, bony fishes, archosauriforms, lepidosauromorphs, cynodonts and other reptiles. Based on its fossil flora and fauna, the Tiki Formation is globally correlated with other coeval horizons such as the lower member of the Maleri Formation of the Pranhita–Godavari basin, the upper part of the Santa Maria Formation of Brazil, the Camp Springs and lower Tecovas formations of the Chinle Group, USA.