Metabolism of Biochanin a and Formononetin by Human Liver Microsomes in Vitro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Note High-Performance Liquid Chromatography
Journal of Chromatography. 234 (1982) 494--496 Elsevier Scientific Publishing Company, Amsterdam - Printed in The Netherlands CHROM. 14.241 Note High-performance liquid chromatography separation of soybean iso flavones and their glucosides A. C. ELDRIDGE Nonhern Regional Research Celller. Agricll/Illra/ Research. Science and Edllcmion Administration, U.S. Department of Agricll/llIre, 1815 North University Street, Peoria. 1L 61604 (U.S.A.; (Received July 27th, 1981) Soybeans are known to contain several isoflavones (daidzein, glycitein, genis tein) and isoflavone glucosides l (daidzin, glycitein-7-f3-0-glucoside, genistin) which 2 3 4 have been reported to have estrogenic , antifungal and antioxidant activity. The quantitative determination of soybean isoflavones has been reported by gas-liquid chromatography (GLC)l, and more recently high-performance liquid chromatogra phy (HPLC)5.6.7 has been used. Carlson and Dolphin5 separated soybean isoflavone aglycones present in an alcohol extract on a pPorasil column. The isoflavone glucosides were determined after hydrolytic treatment with aqueous acid. In a paper by West et al. 6 only two isoflavone aglycones from soybean extracts were separated. This communication reports the development of a procedure for the separation of the naturally occurring soybean isoflavone glucosides and isoflavone algycones using HPLC with mild solvents and reversed phase packing that may be less likely to catalyze decomposition compared to other packings. MATERIALS AND METHODS* A Waters Assoc. (Milford, MA, U.S.A.) HPLC system was used, comprised of a WISP 710A, M-45 solvent delivery system, Model 660 solvent flow programmer, Model 450 variable-wavelength detector, with a DuPont Zorbax ODS 25 x 0.46 cm 1.0. -

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Content and Composition of Isoflavonoids in Mature Or Immature
394 Journal of Health Science, 47(4) 394–406 (2001) Content and Composition of tivities1) and reported to be protective against can- cer, cardiovascular diseases and osteoporosis.3–9) Isoflavonoids in Mature or Much research has been reported about the content Immature Beans and Bean of isoflavonoids in soybeans and soybean-derived processed foods.10–23) In contrast, there are few re- Sprouts Consumed in Japan ports about the isoflavonoid content in beans other than soybeans.11,12,18,23) Yumiko Nakamura,* Akiko Kaihara, Japanese people are reported to ingest Kimihiko Yoshii, Yukari Tsumura, isoflavonoids mainly through the consumption of Susumu Ishimitsu, and Yasuhide Tonogai soybeans and its derived processed foods.20) Re- cently, we estimated that the Japanese daily intake Division of Food Chemistry, National Institute of Health Sci- of isoflavonoids from soybeans and soybean-based ences, Osaka Branch, 1–1–43 Hoenzaka, Chuo-ku, Osaka 540– processed foods is 27.80 mg per day (daidzein 0006, Japan (Received January 9, 2001; Accepted April 6, 2001) 12.02 mg, glycitein 2.30 mg and genistein 13.48 mg).24) However, isoflavonoid intake from the The content of 9 types of isoflavonoids (daidzein, consumption of immature beans, sprouts and beans glycitein, genistein, formononetin, biochanin A, other than soybeans has not been elucidated. Here coumestrol, daidzin, glycitin and genistin) in 34 do- we have measured the content of isoflavonoids in mestic or imported raw beans including soybeans, 7 mature and immature beans and bean sprouts con- immature beans and 5 bean sprouts consumed in Ja- sumed in Japan, and have compared the content and pan were systematically analyzed. -

Interpreting Sources and Endocrine Active Components of Trace Organic Contaminant Mixtures in Minnesota Lakes
INTERPRETING SOURCES AND ENDOCRINE ACTIVE COMPONENTS OF TRACE ORGANIC CONTAMINANT MIXTURES IN MINNESOTA LAKES by Meaghan E. Guyader © Copyright by Meaghan E. Guyader, 2018 All Rights Reserved A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Civil and Environmental Engineering). Golden, Colorado Date _____________________________ Signed: _____________________________ Meaghan E. Guyader Signed: _____________________________ Dr. Christopher P. Higgins Thesis Advisor Golden, Colorado Date _____________________________ Signed: _____________________________ Dr. Terri S. Hogue Professor and Department Head Department of Civil and Environmental Engineering ii ABSTRACT On-site wastewater treatment systems (OWTSs) are a suspected source of widespread trace organic contaminant (TOrC) occurrence in Minnesota lakes. TOrCs are a diverse set of synthetic and natural chemicals regularly used as cleaning agents, personal care products, medicinal substances, herbicides and pesticides, and foods or flavorings. Wastewater streams are known to concentrate TOrC discharges to the environment, particularly accumulating these chemicals at outfalls from centralized wastewater treatment plants. Fish inhabiting these effluent dominated environments are also known to display intersex qualities. Concurrent evidence of this phenomenon, known as endocrine disruption, in Minnesota lake fish drives hypotheses that OWTSs, the primary form of wastewater treatment in shoreline residences, may contribute to TOrC occurrence and the endocrine activity in these water bodies. The causative agents specific to fish in this region remain poorly understood. The objective of this dissertation was to investigate OWTSs as sources of TOrCs in Minnesota lakes, and TOrCs as potential causative agents for endocrine disruption in resident fish. -

Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity
toxins Article Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity Massimo Picardo 1 , Oscar Núñez 2,3 and Marinella Farré 1,* 1 Department of Environmental Chemistry, IDAEA-CSIC, 08034 Barcelona, Spain; [email protected] 2 Department of Chemical Engineering and Analytical Chemistry, University of Barcelona, 08034 Barcelona, Spain; [email protected] 3 Serra Húnter Professor, Generalitat de Catalunya, 08034 Barcelona, Spain * Correspondence: [email protected] Received: 5 October 2020; Accepted: 26 November 2020; Published: 28 November 2020 Abstract: This study presents the application of a suspect screening approach to screen a wide range of natural toxins, including mycotoxins, bacterial toxins, and plant toxins, in surface waters. The method is based on a generic solid-phase extraction procedure, using three sorbent phases in two cartridges that are connected in series, hence covering a wide range of polarities, followed by liquid chromatography coupled to high-resolution mass spectrometry. The acquisition was performed in the full-scan and data-dependent modes while working under positive and negative ionisation conditions. This method was applied in order to assess the natural toxins in the Ter River water reservoirs, which are used to produce drinking water for Barcelona city (Spain). The study was carried out during a period of seven months, covering the expected prior, during, and post-peak blooming periods of the natural toxins. Fifty-three (53) compounds were tentatively identified, and nine of these were confirmed and quantified. Phytotoxins were identified as the most frequent group of natural toxins in the water, particularly the alkaloids group. -

Analysis of Bovine Serum Albumin Ligands from Puerariae Flos Using Ultrafiltration Combined with HPLC-MS
Hindawi Publishing Corporation Journal of Chemistry Volume 2015, Article ID 648361, 6 pages http://dx.doi.org/10.1155/2015/648361 Research Article Analysis of Bovine Serum Albumin Ligands from Puerariae flos Using Ultrafiltration Combined with HPLC-MS Ping Tang,1,2 Shihui Si,1 and Liangliang Liu3 1 College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, China 2School of Environmental Science and Engineering, Hubei Polytechnic University, Hubei Key Laboratory of Mine Environmental Pollution Control and Remediation, Huangshi, Hubei 435003, China 3Institute of Bast Fiber Crops, Chinese Academy of Agricultural Sciences, Changsha 410205, China Correspondence should be addressed to Liangliang Liu; [email protected] Received 24 September 2015; Revised 26 November 2015; Accepted 30 November 2015 Academic Editor: Eulogio J. Llorent-Martinez Copyright © 2015 Ping Tang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Rapid screening techniques for identification of active compounds from natural products are important not only for clarification of the therapeutic material basis, but also for supplying suitable chemical markers for quality control. In the present study, ultrafiltration combined with high performance liquid chromatography-mass spectrometry (HPLC-MS) was developed and conducted to screen and identify bovine serum albumin (BSA) bound ligands from Puerariae flos. Fundamental parameters affecting the screening like incubation time, BSA concentration, pH, and temperature were studied and optimized. Under the optimum conditions, nine active compounds were identified by UV and MS data. The results indicated that this method was able to screen and identify BSA bound ligands form natural products without the need of preparative isolation techniques. -

Nutraceuticals Brian Lockwood
CjigVXZji^XVah HZXdcYZY^i^dc 7g^VcAdX`lddY 00 Prelim 2/3/07 18:51 Page i Nutraceuticals 00 Prelim 2/3/07 18:51 Page ii 00 Prelim 2/3/07 18:51 Page iii Nutraceuticals A guide for healthcare professionals Second edition Brian Lockwood BPharm, PhD, MRPharmS Senior Lecturer in Pharmacy, School of Pharmacy and Pharmaceutical Sciences, University of Manchester, Manchester, UK London • Chicago 00 Prelim 2/3/07 18:51 Page iv Published by the Pharmaceutical Press An imprint of RPS Publishing 1 Lambeth High Street, London SE1 7JN, UK 100 South Atkinson Road, Suite 200, Grayslake, IL 60030–7820, USA © Pharmaceutical Press 2007 is a trade mark of RPS Publishing RPS Publishing is the publishing organisation of the Royal Pharmaceutical Society of Great Britain First edition published 2002 Second edition published 2007 Typeset by Type Study, Scarborough, North Yorkshire Printed in Great Britain by TJ International, Padstow, Cornwall ISBN 978 0 85369 659 9 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without the prior written permission of the copyright holder. The publisher makes no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. The right of Brian Lockwood to be identified as the author of this work has been asserted by him in accordance with sections 77 and 78 and subject to section 79(6) of the Copyright, Designs and Patents Act, 1988. -

1 1 2 3 4 5 6 7 Bioactive Phenolic Compounds of Soybean
* Manuscript Click here to view linked References 1 Bioactive phenolic compounds of soybean ( Glycine max cv. Merit): modifications by 2 different microbial fermentations 3 4 5 M. Dueñas 2; T. Hernández 1; S. Robredo 1; *I. Estrella 1; R. Muñoz 1 6 7 1Instituto de Fermentaciones Industriales. C.S.I.C. Juan de la Cierva 3, 28006-Madrid. Spain. Fax: 34-915644853. 8 [email protected] 9 2Grupo de Investigación en Polifenoles, Unidad de Nutrición y Bromatología, Facultad de Farmacia, Universidad de 10 Salamanca. Campus Miguel de Unamuno. 37007 Salamanca. Spain. 11 12 *Corresponding author 13 14 Running title: Changes in phenolic compounds of soybeans by microbial fermentations 15 16 17 Abstract 18 Phenolic compounds of soybeans ( Glycine max ) are partially responsible of their beneficial 19 health effects. The most abundant phenolics in soybeans are isoflavones, mainly daidzein, 20 glycitein, and genistein and several derivatives of them. Other phenolic compounds are also 21 present but they have been minimally studied. In this work, the soybean seeds were fermented by 22 the microbiota present on the seeds or by inoculation with Aspergillus oryzae, Rhizopus oryzae or 23 Bacillus subtilis . The effect of microbial fermentation on the phenolic composition of soybeans 24 was studied. All the assayed microorganisms brought out variations in phenolics. An increase in 25 the concentration of phenolic acids was observed. Flavonoids were differently metabolized 26 depending on the microorganism used. In all samples the most important changes were produced 27 in the isoflavones content, observing an increase on the aglycones, glycitein, daidzein and 28 genistein. 29 30 Key words : soybean, fermentation, phenolic compounds, isoflavones 31 1 32 1. -

Study Protocol and Statistical Analysis Plan
Confidential Clinical study protocol number: J1228 Page 1 Version Date: May 7, 2018 IRB study Number: NA_00067315 A Trial of maintenance Rituximab with mTor inhibition after High-dose Consolidative Therapy in CD20+, B-cell Lymphomas, Gray Zone Lymphoma, and Hodgkin’s Lymphoma Principal Investigator: Douglas E. Gladstone, MD The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins 1650 Orleans Street, CRBI-287 Baltimore, MD 21287 Phone: 410-955-8781 Fax: 410-614-1005 Email: [email protected] IRB Protocol Number: NA_00067315 Study Number: J1228 IND Number: EXEMPT Novartis Protocol Number: CRAD001NUS157T Version: May 7, 2018 Co-Investigators: Jonathan Powell 1650 Orleans Street, CRBI-443 Phone: 410-502-7887 Fax: 443-287-4653 Email: [email protected] Richard Jones 1650 Orleans Street, CRBI-244 Phone: 410-955-2006 Fax: 410-614-7279 Email: [email protected] Confidential Clinical study protocol number: J1228 Page 2 Version Date: May 7, 2018 IRB study Number: NA_00067315 Satish Shanbhag Johns Hopkins Bayview Medical Center 301 Building, Suite 4500 4940 Eastern Ave Phone: 410-550-4061 Fax: 410-550-5445 Email: [email protected] Statisticians: Gary Rosner Phone: 410-955-4884 Email: [email protected] Marianna Zahurak Phone: 410-955-4219 Email: [email protected] Confidential Clinical study protocol number: J1228 Page 3 Version Date: May 7, 2018 IRB study Number: NA_00067315 Table of contents Table of contents ......................................................................................................................... 3 List of abbreviations -

Memorandum Date: June 6, 2014
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Memorandum Date: June 6, 2014 From: Bisphenol A (BPA) Joint Emerging Science Working Group Smita Baid Abraham, M.D. ∂, M. M. Cecilia Aguila, D.V.M. ⌂, Steven Anderson, Ph.D., M.P.P.€* , Jason Aungst, Ph.D.£*, John Bowyer, Ph.D. ∞, Ronald P Brown, M.S., D.A.B.T.¥, Karim A. Calis, Pharm.D., M.P.H. ∂, Luísa Camacho, Ph.D. ∞, Jamie Carpenter, Ph.D.¥, William H. Chong, M.D. ∂, Chrissy J Cochran, Ph.D.¥, Barry Delclos, Ph.D.∞, Daniel Doerge, Ph.D.∞, Dongyi (Tony) Du, M.D., Ph.D. ¥, Sherry Ferguson, Ph.D.∞, Jeffrey Fisher, Ph.D.∞, Suzanne Fitzpatrick, Ph.D. D.A.B.T. £, Qian Graves, Ph.D.£, Yan Gu, Ph.D.£, Ji Guo, Ph.D.¥, Deborah Hansen, Ph.D. ∞, Laura Hungerford, D.V.M., Ph.D.⌂, Nathan S Ivey, Ph.D. ¥, Abigail C Jacobs, Ph.D.∂, Elizabeth Katz, Ph.D. ¥, Hyon Kwon, Pharm.D. ∂, Ifthekar Mahmood, Ph.D. ∂, Leslie McKinney, Ph.D.∂, Robert Mitkus, Ph.D., D.A.B.T.€, Gregory Noonan, Ph.D. £, Allison O’Neill, M.A. ¥, Penelope Rice, Ph.D., D.A.B.T. £, Mary Shackelford, Ph.D. £, Evi Struble, Ph.D.€, Yelizaveta Torosyan, Ph.D. ¥, Beverly Wolpert, Ph.D.£, Hong Yang, Ph.D.€, Lisa B Yanoff, M.D.∂ *Co-Chair, € Center for Biologics Evaluation & Research, £ Center for Food Safety and Applied Nutrition, ∂ Center for Drug Evaluation and Research, ¥ Center for Devices and Radiological Health, ∞ National Center for Toxicological Research, ⌂ Center for Veterinary Medicine Subject: 2014 Updated Review of Literature and Data on Bisphenol A (CAS RN 80-05-7) To: FDA Chemical and Environmental Science Council (CESC) Office of the Commissioner Attn: Stephen M. -
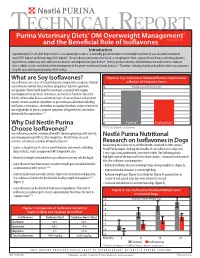
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

Changes of Phytoestrogens Daidzein, Genistein and Their Glycosides Daidzin and Genistin and Coumestrol During Processing of Soyabeans
Czech J. Food Sci. Vol. 22, Special Issue Changes of Phytoestrogens Daidzein, Genistein and Their Glycosides Daidzin and Genistin and Coumestrol during Processing of Soyabeans J. LOJZA*, V. SCHULZOVÁ and J. HAJŠLOVÁ Department of Food Chemistry and Analysis, Institute of Chemical Technology, Prague, Czech Republic, *E-mail: [email protected] Abstract: Phytoestrogens represent biologically active compounds showing estrogenic activity similar to that of sex hormones – estrogens. Various adverse effects such as sterility, increase of females’ genitals, lost of males’ copulation activity, etc. were observed in farm animals after exposure to higher amounts of fodder containing phytoestrogens. On the other side, their presence in human diet is nowadays the object of many research stud- ies concerned with prevention of breast and prostate cancer, osteoporosis and other hormone-linked diseases by dietary intake of phytoestrogens. Soya (Glycine max) is one of the main sources of these compounds in diet. Isoflavones daidzein and genistein occurring either free or bound in glycosides are the main phytoestrogens in this food crop. Coumesterol representing coumestans is another effective phytoestrogen contained in some ed- dible plants. In the first part of our study, analytical method for determination of free and total phytoestrogens was developed and validated. Following steps are included: (i) acid hydrolysis (only for “total phytoestrogens” analysis), (ii) extraction with methanol/water mixture, (iii) SPE preconcentration; (iv) identification/quantifica- tion using HPLC/DAD/FLD. The aim of present study was to document the fate of phytoestrogens and their forms during household/industrial processing. As documented in our experiments the most dynamic changes of phytoestrogen levels occur during soyabeans sprouting.