Nipah Virus Infection in Bats (Order Chiroptera) in Peninsular Malaysia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ing Items Have Been Registered
ACCEPTANCES Page 1 of 37 June 2017 LoAR THE FOLLOWING ITEMS HAVE BEEN REGISTERED: ÆTHELMEARC Alrekr Bergsson. Device. Per saltire gules and sable, in pale two wolf’s heads erased and in fess two sheaves of arrows Or. Brahen Lapidario. Name and device. Argent, a lozenge gules between six French-cut gemstones in profile, two, two and two azure, a base gules. The ’French-cut’ is a variant form of the table cut, a precursor to the modern brilliant cut. It dates to the early 15th Century, according to "Diamond Cuts in Historic Jewelry" by Herbert Tillander. There is a step from period practice for gemstones depicted in profile. Hrólfr á Fjárfelli. Device. Argent estencely sable, an ash tree proper issuant from a mountain sable. Isabel Johnston. Device. Per saltire sable and purpure, a saltire argent and overall a winged spur leathered Or. Lisabetta Rossi. Name and device. Per fess vert and chevronelly vert and Or, on a fess Or three apples gules, in chief a bee Or. Nice early 15th century Florentine name! Símon á Fjárfelli. Device. Azure, a drakkar argent and a mountain Or, a chief argent. AN TIR Akornebir, Canton of. Badge for Populace. (Fieldless) A squirrel gules maintaining a stringless hunting horn argent garnished Or. An Tir, Kingdom of. Order name Order of Lions Mane. Submitted as Order of the Lion’s Mane, we found no evidence for a lion’s mane as an independent heraldic charge. We therefore changed the name to Order of _ Lions Mane to follow the pattern of Saint’s Name + Object of Veneration. -

The Rhinolophus Affinis Bat ACE2 and Multiple Animal Orthologs Are Functional 2 Receptors for Bat Coronavirus Ratg13 and SARS-Cov-2 3
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.16.385849; this version posted November 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 The Rhinolophus affinis bat ACE2 and multiple animal orthologs are functional 2 receptors for bat coronavirus RaTG13 and SARS-CoV-2 3 4 Pei Li1#, Ruixuan Guo1#, Yan Liu1#, Yintgtao Zhang2#, Jiaxin Hu1, Xiuyuan Ou1, Dan 5 Mi1, Ting Chen1, Zhixia Mu1, Yelin Han1, Zhewei Cui1, Leiliang Zhang3, Xinquan 6 Wang4, Zhiqiang Wu1*, Jianwei Wang1*, Qi Jin1*,, Zhaohui Qian1* 7 NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen 8 Biology, Chinese Academy of Medical Sciences and Peking Union Medical College1, 9 Beijing, 100176, China; School of Pharmaceutical Sciences, Peking University2, 10 Beijing, China; .Institute of Basic Medicine3, Shandong First Medical University & 11 Shandong Academy of Medical Sciences, Jinan 250062, Shandong, China; The 12 Ministry of Education Key Laboratory of Protein Science, Beijing Advanced 13 Innovation Center for Structural Biology, Beijing Frontier Research Center for 14 Biological Structure, Collaborative Innovation Center for Biotherapy, School of Life 15 Sciences, Tsinghua University4, Beijing, China; 16 17 Keywords: SARS-CoV-2, bat coronavirus RaTG13, spike protein, Rhinolophus affinis 18 bat ACE2, host susceptibility, coronavirus entry 19 20 #These authors contributed equally to this work. 21 *To whom correspondence should be addressed: [email protected], 22 [email protected], [email protected], [email protected] 23 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.16.385849; this version posted November 17, 2020. -

Heraldic Terms
HERALDIC TERMS The following terms, and their definitions, are used in heraldry. Some terms and practices were used in period real-world heraldry only. Some terms and practices are used in modern real-world heraldry only. Other terms and practices are used in SCA heraldry only. Most are used in both real-world and SCA heraldry. All are presented here as an aid to heraldic research and education. A LA CUISSE, A LA QUISE - at the thigh ABAISED, ABAISSÉ, ABASED - a charge or element depicted lower than its normal position ABATEMENTS - marks of disgrace placed on the shield of an offender of the law. There are extreme few records of such being employed, and then only noted in rolls. (As who would display their device if it had an abatement on it?) ABISME - a minor charge in the center of the shield drawn smaller than usual ABOUTÉ - end to end ABOVE - an ambiguous term which should be avoided in blazon. Generally, two charges one of which is above the other on the field can be blazoned better as "in pale an X and a Y" or "an A and in chief a B". See atop, ensigned. ABYSS - a minor charge in the center of the shield drawn smaller than usual ACCOLLÉ - (1) two shields side-by-side, sometimes united by their bottom tips overlapping or being connected to each other by their sides; (2) an animal with a crown, collar or other item around its neck; (3) keys, weapons or other implements placed saltirewise behind the shield in a heraldic display. -
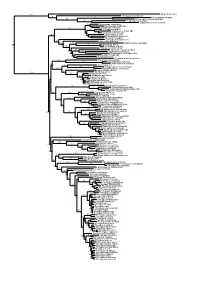
Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

THE IRON MAN Ted Hughes Chapter 5 the Iron Man's Challenge There
THE IRON MAN Ted Hughes Chapter 5 The Iron Man’s Challenge There was no time to be wasted. The Iron Man allowed himself to be taken to pieces, arms, legs, body, head, all separate, so each part could be flown to Australia on a different airliner. He was too big to be flown out in one piece. At the same time a ship sailed from China, loaded with great iron girders, and another ship sailed from Japan loaded with fuel oil. The Iron Man had ordered these. The girders and the oil and a team of engineers were unloaded on the beach of Northern Australia, near the space-bat-angel-dragon’s neck. Then the Iron Man’s parts were landed at the same spot, and the engineers fitted him together. He stood up on the beach and shouted his challenge. “Sit up,” he roared. “Sit up and take notice, you great space-lizard.” The space-bat-angel-dragon sat up slowly. He had never noticed the fussing of the boats and aeroplanes down there on the beach near his neck. Now he gazed in surprise at the Iron Man, who seemed very tiny to him, though his voice was big enough. The Iron Man spoke again. “I challenge you,” he shouted, “to a test of strength.” A test of strength? The space-bat-angel-dragon couldn’t believe his ears. A tiny little creature like the Iron Man challenging him to a test of strength? He simply laughed. Loud and long. Then he peered down again at the Iron Man, while the echo of his laugh was still rolling round the earth. -

Conventional Wisdom on Roosting Behaviour of Australian
Conventional wisdom on roosting behaviour of Australian flying foxes - a critical review, and evaluation using new data Tamika Lunn1, Peggy Eby2, Remy Brooks1, Hamish McCallum1, Raina Plowright3, Maureen Kessler4, and Alison Peel1 1Griffith University 2University of New South Wales 3Montana State University 4Montana State University System April 12, 2021 Abstract 1. Fruit bats (Family: Pteropodidae) are animals of great ecological and economic importance, yet their populations are threatened by ongoing habitat loss and human persecution. A lack of ecological knowledge for the vast majority of Pteropodid bat species presents additional challenges for their conservation and management. 2. In Australia, populations of flying-fox species (Genus: Pteropus) are declining and management approaches are highly contentious. Australian flying-fox roosts are exposed to management regimes involving habitat modification, either through human-wildlife conflict management policies, or vegetation restoration programs. Details on the fine-scale roosting ecology of flying-foxes are not sufficiently known to provide evidence-based guidance for these regimes and the impact on flying-foxes of these habitat modifications is poorly understood. 3. We seek to identify and test commonly held understandings about the roosting ecology of Australian flying-foxes to inform practical recommendations and guide and refine management practices at flying-fox roosts. 4. We identify 31 statements relevant to understanding of flying-fox roosting structure, and synthesise these in the context of existing literature. We then contribute contemporary data on the fine-scale roosting structure of flying-fox species in south-eastern Queensland and north- eastern New South Wales, presenting a 13-month dataset from 2,522 spatially referenced roost trees across eight sites. -

NHBSS 042 1O Robinson Obs
Notes NAT. NAT. HIST. B 肌 L. SIAM Soc. 42: 117-120 , 1994 Observation Observation on the Wildlife Trade at the Daily Market in Chiang Kh an , Northeast Thailand In In a recent study into the wildlife trade between Lao PDR. and Thailand S即 KOSAMAT 組 A et al. (1 992) found 伽 tit was a major 伽 eat to wildlife resources in Lao. Th ey surveyed 15 locations along 白e 百凶 Lao border ,including Chiang Kh an. Only a single single visit was made to the market in Chiang Kh佃, on 8 April 1991 , and no wildlife products products were found. τbe border town of Chi 佃 g Kh an , Loei Pr ovince in northeast 百lailand is situated on the the banks of the Mekong river which sep 釘 ates Lao PD R. and 官 lailand. In Chiang Kh an there there is a small market held twice each day. Th e market ,situated in the centre of town , sells sells a wide r佃 ge of products from f記sh fruit ,vegetables , meat and dried fish to clothes and and household items. A moming market begins around 0300 h and continues until about 0800 h. The evening market st 紅白紙 1500 h and continues until about 1830 h. During During the period November 1992 to November 1993 visits were made to Chiang Kh佃 eve 凶ng market , to record the wildlife offered for sale. Only the presence of mam- mals ,reptiles and birds was recorded. Although frogs ,insects ,fish , crabs and 加 t eggs , as as well as meat 仕om domestic animals , were sold ,no record of this was made. -

20' Up! Making and Flying Silk War Standards
20’ Feet Up! Making and Flying Silk War Standards. Baroness Theodora Delamore and Baron Alan Gravesend, UofA#73, Oct 2008. 20’ Up! Making and Flying silk war standards Baroness Theodora Delamore, OP and Baron Alan Gravesend, OP October 6, 2008. University of Atlantia, Session#73 Course outline • The language of vexillology in general, and the anatomy of standards in particular • Historical examples of war standards (or not many cloth things survive). • Designing your own war standards (while there are no cut and dry rules, there’s a general trend) • Basic silk painting skills necessary for producing your own war standard • A flag pole all your own (or 20’ up!) Vexillology: The study of flags Finial Banner: A flag that generally corresponds exactly to a coat of arms. Finial: (often decorative) top of the flag staff. Fly: Part of a flag opposite the staff (sinister) Hoist Fly Gonfalon: Flag hung from a crossbar Ground: the field of the flag. Width Hoist: part of the flag nearest the staff (dexter) Livery colors: principal tinctures of a coat of Length Staff arms; typically a color and metal Pencel, Pennon: small flag, such as a lance Anatomy of a Flag (W. Morgenstern) flag; armirgeous. Often bears a badge against livery colors. (Modern version = pennant.) Pennoncelle: long, narrow pennon or streamer. Often single pointed. Pinsil: Scotish pennon, featuring the owner’s crest & motto in the hoist and badge in the fly Schwenkel: a single long tail extended from the upper fly corner of a flag. Standard: long, tapering flag of heraldic design. Swallow tailed – having a large triangular section cut out of the fly end. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

A Checklist of the Mammals of South-East Asia
A Checklist of the Mammals of South-east Asia A Checklist of the Mammals of South-east Asia PHOLIDOTA Pangolin (Manidae) 1 Sunda Pangolin (Manis javanica) 2 Chinese Pangolin (Manis pentadactyla) INSECTIVORA Gymnures (Erinaceidae) 3 Moonrat (Echinosorex gymnurus) 4 Short-tailed Gymnure (Hylomys suillus) 5 Chinese Gymnure (Hylomys sinensis) 6 Large-eared Gymnure (Hylomys megalotis) Moles (Talpidae) 7 Slender Shrew-mole (Uropsilus gracilis) 8 Kloss's Mole (Euroscaptor klossi) 9 Large Chinese Mole (Euroscaptor grandis) 10 Long-nosed Chinese Mole (Euroscaptor longirostris) 11 Small-toothed Mole (Euroscaptor parvidens) 12 Blyth's Mole (Parascaptor leucura) 13 Long-tailed Mole (Scaptonyx fuscicauda) Shrews (Soricidae) 14 Lesser Stripe-backed Shrew (Sorex bedfordiae) 15 Myanmar Short-tailed Shrew (Blarinella wardi) 16 Indochinese Short-tailed Shrew (Blarinella griselda) 17 Hodgson's Brown-toothed Shrew (Episoriculus caudatus) 18 Bailey's Brown-toothed Shrew (Episoriculus baileyi) 19 Long-taied Brown-toothed Shrew (Episoriculus macrurus) 20 Lowe's Brown-toothed Shrew (Chodsigoa parca) 21 Van Sung's Shrew (Chodsigoa caovansunga) 22 Mole Shrew (Anourosorex squamipes) 23 Himalayan Water Shrew (Chimarrogale himalayica) 24 Styan's Water Shrew (Chimarrogale styani) Page 1 of 17 Database: Gehan de Silva Wijeyeratne, www.jetwingeco.com A Checklist of the Mammals of South-east Asia 25 Malayan Water Shrew (Chimarrogale hantu) 26 Web-footed Water Shrew (Nectogale elegans) 27 House Shrew (Suncus murinus) 28 Pygmy White-toothed Shrew (Suncus etruscus) 29 South-east -

Rabies Bulletin Europe
RABIES BULLETIN EUROPE Volume 22/No 1 Quarter 1 1998 Contents Page 1. Introduction 3 2. Summary of Rabies in Europe 3-4 3. Rabies in Individual Countries 4-8 4. Miscellaneous Article 4.1 Global timely access to country related data on rabies diagnosis, prevention and control 9-11 5. Rabies Case Data Europe 5.1 Table 5.1 : 1. Quarter 1998 12 5.2 Table 5.2: Other Animal Species, 1. Quarter 1998 13 5.3 Tables: Individual Countries, 1. Quarter 1998 14-23 6. Ust of Contributors 24 7. Annexes Map of Rabies Cases In Russia, 1. Quarter 1998 Annex 1 Map of Rabies Cases In Turkey, 1. Quarter 1998 Annex 2 Map of Rabies Cases In Europe, 1. Quarter 1998 Annex 3 The Rabies Bulletin Europe has been compiled and edited by the WHO Collaborating Centre for Rabies Surveillance & Research at the Federal Research Centre for Virus Diseases of Animals Postfach (P.O.Box) 1149 D-72001 Tiibingen Federal Republic of Germany Dr. W.W. Muller Phone (0)-7071-967-210 Dr. J.H. Cox Phone (0)-7071-967-226 K.-P. Hohnsbeen, Data Proce&&ing Fax (0)-7071-967-303 e-mail WHO-RABIF.SiiYl'UE. BFAV.DE page2 Rabies Bulletin Europe- Vol 22/No 111998 The.Rabies Bulletin Europe is sponsored by the World Health Organization, Geneva, and the International Office of Epizootics, Paris Gratefully acknowledged is the financial support of the WHO Collaborating Centre by the Bundesministerium fur Gesundheit · Bono - Bad Godesberg 2 1st Quarter: January -March 1998 page 3 1. INTRODUCTION This BULLETIN des gularly yet.