WHO Guidelines on Good Herbal Processing Practices for Herbal Medicines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chef in Residence Recipes
CHEF IN RESIDENCE RECIPES CHEF SHOLA OLUNYOLO OTTO FILE CORN GRITS 3 CARROT SALAD 4 EGUSI SOUP 7 GOAT PEPPER SOUP 8 CHEF OMAR TATE HOPPIN’ JOHN 12 CHEF JOHNNY ORTIZ FLOUR TORTILLAS 16 RED POSOLE 17 CHEF SHOLA OLUNLOYO JANUARY 13 - FEBRUARY 6 SHOLA OLUNLOYO AT STONE AT “In Nigeria, food is the focal point BARNS of every celebration, as much for nourishment as for joy. These recipes, informational videos and more highlight the cultural foodways at the heart of Nigerian community—and also integrate the knowledge and technique from my personal journey as a chef through Southeast Asia, East Asia, Europe and West Africa. My cuisine is not competing with tradition; it’s an evolution of tradition.” From January 13 to February 6, Chef Shola Olunloyo executed his residency at Stone Barns as our first resident in a series of four. He explored Yoruba Southwest Nigerian cuisine, while highlighting differences and similarities among global cuisines. After cooking through some of the toughest kitchens in the industry, Philadelphia-based chef Shola Olunloyo has spent the two decades with his experimental project, Studiokitchen, a kitchen lab where he plays with food and equipment to enhance his understanding of culinary arts and develop projects for restaurants and foodservice manufacturers. At Stone Barns, he explored farm ingredients from goat to Otto File corn, bringing a flavor- forward approach with extensive fermentation. The residency was supported by Chef Bill Yosses, former White House Executive Pastry Chef during the Bush and Obama administrations, who collaborated with Shola for the residency’s West African influenced pastry program. -

IE Decoction Instructions
Inner Ecology Decoction Instructions What tools might I require? Always use glass, ceramic, and stainless steel tools. Do not use plastic, aluminum, or coated cookware or utensils. 1. Water, preferably filtered 2. Stovetop or slow cooker 3. Large pot with lid (around 3 qt.) 4. Strainer 5. Bowl 6. Liquid measuring cup Your formula 7. Small pot with a lid (around 1 qt.), if your formula contains any medicinal requires a small pot. that requires separate decoction A “decoction” is a liquid extract made by boiling medicinals in water. Making a decoction is like making tea, but stronger, as the herbs are simmered in the water, rather than just steeping. Making a Basic Decoction Your formula 1. Read all instructions received with your formula before cooking. contains herbs that • If your formula contains smaller, labeled bags, remove these from the require special primary formula and set aside. These are herbs that require special preparation. preparations. Please see below for detailed instructions. • If your formula contains unlabeled teabags, leave these unopened in the primary formula. Unlabeled teabags do not require special preparation. 2. Place your primary formula (all medicinals not in specialized bags) into your large pot. Add enough water to keep the herbs well covered, and soak for 30 minutes. Make sure to have enough water to keep the herbs covered throughout the cook process; you may add water if needed. Simmer your 3. With the lid in place, bring your medicinals to a boil. Reduce to a simmer formula for ____ for 30-60 minutes, according to your herbalist’s instructions. -

The Sustainer of Human Life 20 Mekong River
THE SUSTAINER OF HUMAN LIFE Water is, of course, fundamental to life, but those of us Wat in Cambodia or Wat Phou in Laos. Or it may mean who live in cities, where water comes from a tap and dredging channels and building dikes, as in the Mekong food comes from a supermarket, can easily forget how Delta, to handle the immense floods that inundate the heavily human life depends on a regular supply of water. area in the rainy season. In the Mekong region, water from rainfall or diverted Important as it is to agriculture, water is equally vi from rivers into irrigation systems sustains rice fields, tal for countless varieties of fish, mammals, crustaceans, vegetable gardens, fruit plantations, and bamboo groves. mollusks, and amphibians that, together with the staple The immense plains of Northeast Thailand, much of rice, are mainstays of the diet of Mekong residents. Cambodia, and the Mekong Delta of Vietnam are the Before rice is planted, the flooded paddies teem with world's rice bowl. The peoples living in the region have small fish, snails, crabs, and frogs, and children are sculpted the surface of the land to bring water to rice often sent out to the fields to catch the evening meal. crops. Upriver, this may mean constructing elaborate In streams, ponds, and rivers, larger fish are caught in irrigation systems with waterwheels to bring water out all kinds of nets and a dizzying variety of traps. Recent of rivers and into paddies. Downriver, it may involve decades have seen intensive aquaculture in the region. -

Alchemist's Handbook-First Edition 1960 from One to Ten
BY THE SAME AUTHOR wqt Drei NoveIlen (German) 1932 The Alchemist's Handbook-First Edition 1960 From One to Ten . .. .. 1966 Alrqtuttaf!i Praxis Spagyrica Philosophica 1966 The Seven Rays of the Q.B.L.-First Edition 1968 Praetische Alchemie irn Zwanzigsten Jahrundert 1970 ~aubhnnk (Practical Alchemy in the 20th Century-German) Der Mensch und die kosmischen Zyklen (German) 1971 (Manual for Practical Laboratory Alchemy) Men and the Cycles of the Universe 1971 Von Eins bis Zehn (From One to Ten-German) 1972 El Hombre y los Ciclos del Universo (Spanish) 1972 by Die Sieben Strahlen der Q.B.L. 1973 (The Seven Rays of the Q.B.L.-German) FRATER ALBERTUS SAMUEL WEISER New York CONTENTS Foreword 6 Preface to the First Edition 10 Preface to the Second Revised Edition 13 Chapter I Introduction to Alchemy 14 Samuel Weiser, Inc. Chapter 11 740 Broadway The Lesser Circulation 24 New York, N.Y. 10003 Chapter III First Published 1960 The Herbal Elixir Revised Edition 1974 Chapter IV Third Printing 1978 Medicinal Uses 43 Chapter V © 1974 Paracelsus Research Society Herbs and Stars 47 Salt Lake City, Utah, U.S.A. Chapter VI Symbols in Alchemy 56 ISBN 0 87728 181 5 Chapter VII Wisdom of the Sages 65 Conclusion 100 Alchemical Manifesto 120 ILLUSTRATIONS On the Way to the Temple 5 Soxhlet Extractor 34 Basement Laboratory 41 Essential Equipment 42 Printed in U.S.A. by Qabalistic Tree of Life 57 NOBLE OFFSET PRINTERS, INC. NEW YORK, N.Y. 10003 Alchemical Signs 58 ORIGINAL OIL PAINTING AT PARACELSUS RESEARCH SOCIETY .. -

Network Pharmacology and Traditional Chinese Medicine: Devel- Opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1*
ISSN: 2377-3634 Lu et al. Int J Diabetes Clin Res 2017, 4:077 DOI: 10.23937/2377-3634/1410077 Volume 4 | Issue 2 International Journal of Open Access Diabetes and Clinical Research REVIEW ARTICLE Network Pharmacology and Traditional Chinese Medicine: Devel- opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1* 1College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China 2College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China Check for 3College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China updates *Corresponding author: Yitao Chen, MD, College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected]; Changyu Li, MD, College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected] Abstract Research and Development Dilemma for Anti- Diabetes Drugs Partly due to the failure of single-target drugs, diabetes mel- litus, a chronic metabolic disease with complex pathogene- Type 2 Diabetes Mellitus (T2DM), generally agreed sis and long-term medication requirements, is increasing in to be caused by insulin resistance and/or insulin defi- prevalence worldwide and urgently needs multi-component and multi-target treatments. Traditional Chinese herbs are ciency, constitutes almost 95 percent of all diabetes the principal drug of Chinese medicine, which is effective cases [4]. Because of the pathogenesis, the mainstre- against diabetes. However, Chinese herbs’ mechanism of am anti-diabetic drugs are insulin secretagogues (sul- action is difficult to elucidate due to its multiple components phonylureas and meglitinide analogues), insulin sensi- and multi-target effects. -

Supplementary Materials 1
Supplementary materials 1 Table S1 The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Botanical Pin Yin Name Form Ingredients Recommendation for daily intake (g) preparations (汉语) Plant food supplements (PFS) Si Ji Kang Mei Yang Xin Yuan -Rou Dou Kou xylooligosaccharide, isomalt, nutmeg (myristica PFS 1 Fu He Tang Pian tablet 4 tablets (1.4 g) fragrans), galangal, cinnamon, chicken gizzards (四季康美养心源-肉豆蔻复合糖片) Ai Si Meng Hui Xiang fennel seed, figs, prunes, dates, apples, St.Johns 2-4 tablets (2.8-5.6 g) PFS 2 Fu He Pian tablet Breed, jamaican ginger root (爱司盟茴香复合片) Zi Ran Mei Xiao Hui Xiaong Jiao Nang foeniculi powder, cinnamomi cortex, papaya PFS 3 capsule concentrated powder, green oat concentrated powder, 3 capsules (1.8 g) (自然美小茴香胶囊) brewer’s yeast, cabbage, monkey head mushroom An Mei Qi Hui Xiang Cao Ben Fu He Pian fennel seed, perilla seed, cassia seed, herbaceous PFS 4 tablet 1-2 tablets (1.4-2.8 g) (安美奇茴香草本复合片) complex papaya enzymes, bromelain enzymes, lactobacillus An Mei Qi Jiao Su Xian Wei Ying Yang Pian acidophilus, apple fiber, lemon plup fiber, fennel PFS 5 tablet seed, cascara sagrada, jamaican ginger root, herbal 2 tablets (2.7 g) (安美奇酵素纤维营养片) support complex (figs, prunes, dates, apples, St. Johns bread) Table S1 (continued) The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Pin Yin Name Botanical Form Ingredients Recommendation for daily intake (g) preparations (汉语) Gan Cao Pian glycyrrhiza uralensis, licorice -

Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Publications 1-18-2012 Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats Zhiying Dou Tianjin University of Traditional Chinese Medicine Kefeng Li Michigan Technological University Ping Wang Tianjin University of Traditional Chinese Medicine Liu Cao Tianjin University of Traditional Chinese Medicine Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Recommended Citation Dou, Z., Li, K., Wang, P., & Cao, L. (2012). Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules, 17(1), 951-970. http://doi.org/10.3390/molecules17010951 Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/1969 Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Molecules 2012, 17, 951-970; doi:10.3390/molecules17010951 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats Zhiying Dou 1,*, Kefeng Li 2, Ping Wang 1 and Liu Cao 1 1 College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China 2 Department of Biological Sciences, Michigan Technological University, Houghton, MI 49931, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-22-5959-6235. Received: 29 November 2011; in revised form: 5 January 2012 / Accepted: 9 January 2012 / Published: 18 January 2012 Abstract: Vinegar and wine processing of medicinal plants are two traditional pharmaceutical techniques which have been used for thousands of years in China. -
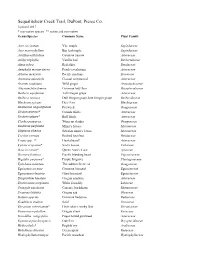
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

WHMF123 Herbal Botany Last Modified: 16-Apr-2021
SUBJECT OUTLINE Subject Name: Subject Code: Herbal Botany WHMF123 SECTION 1 – GENERAL INFORMATION Award/s: Total Course Credit Points: Level: Bachelor of Complementary Medicine 48 3rd Year Duration: 1 Semester Subject is: Elective Subject Credit Points: 2 Student Workload: No. timetabled hours per week: No. personal study hours per week: Total hours per week: 3 2 5 Delivery Mode*: ☐ On campus ☒ Online / Digital ☐ Blended ☐ Intensive Weekly Session^ Format/s - 1 session per week: ☒ eLearning modules: Lectures: Narrated PowerPoint presentations Tutorials: can include asynchronous tutor moderated discussion forum and activities, learning journal activities or other web-based resources *All modes are supported by the online learning management system which will include subject documents such as handouts, readings and assessment guides. ^A ‘session’ is made up of 3 hours of timetabled / online study time per week unless otherwise specified. Each subject has a set number of sessions as outlined above. Study Pattern: ☒ Full Time ☒ Part Time Pre-requisites: Nil Co-requisites: Nil SECTION 2 – ACADEMIC DETAILS Subject Rationale This foundational herbal medicine subject introduces students to the study of plant medicine via an exploration of botany. Through an understanding of basic plant morphology, botanical terminology, taxonomy, and nomenclature, students learn to recognise similar and different physical characteristics of plants and to identify plant specimens. Additionally students are introduced to the legislative and regulatory frameworks that govern the manufacture and sale of botanical medicines in Australia. Australian College of Natural Medicine Pty Ltd trading as Endeavour College of Natural Health, FIAFitnation (National CRICOS #00231G, RTO #31489) WHMF123 Herbal Botany Last modified: 16-Apr-2021 Version: 12.0 Page 1 of 5 Learning Outcomes 1. -

View Article
OPEN ACCESS Freely available online Biology and Medicine Review Article Herbal Prescription for COVID-19 Enqin Zhang* Department of Medicine, UK Academy of Chinese Medicine, United Kindom ABSTRACT The clinical studies from China have proved that the use of herbal medicine has played a significant role in the prevention and treatment of COVID-19. This article aims to introduce the six most effective herbal prescriptions in Traditional Chinese Medicine (TCM) for treating the coronavirus (COVID-19). Each formula has been described in detail including the name, source, indication, ingredients (Chinese Pinyin, English and Latin names), usage and discussion, etc. The first chief formula introduced in this article is the most popular prescription published by The National Health Commission of People’s Republic of China on 3/3 2020 for the treatment and prevention of coronavirus infection and pneumonia; and subsequent formulas are the modified classical herbal prescriptions and my experienced herbal formula I often use in the UK. Keywords: COVID-19; Herbal formulas; Ingredients, Indications; Usage; Discussion INTRODUCTION infected by COVID -19 in the UK. This is why I choose to write this article. The coronavirus (COVID-19) is highly contagious with a characteristic tendency to severely affect the respiratory tract and METHODS the lung in certain individuals .TCM classifies COVID-19 as an epidemic disease termed ‘Wen YI’ and considers both external and Here is detailed information on the anti-coronavirus herbal internal factors contributing to -

City of Vancouver Native Trees and Shrubs Last Revision: 2010 Plant Characteristics (A - M)
City of Vancouver Native Trees and Shrubs Last Revision: 2010 Plant Characteristics (A - M) *This list is representative, but not exhaustive, of the native trees and shrubs historically found in the natural terrestrial habitats of Vancouver, Washington. Botanical Name Common NameGrowth Mature Mature Growth Light / Shade Tolerance Moisture Tolerance Leaf Type Form Height Spread Rate Full Part Full Seasonally Perennially Dry Moist (feet) (feet) Sun Sun Shade Wet Wet Abies grandies grand fir tree 150 40 medium evergreen, 99 999 conifer Acer circinatum vine maple arborescent 25 20 medium deciduous, shrub 99 99 broadleaf Acer macrophyllum bigleaf maple tree 75 60 fast deciduous, 99 999 broadleaf Alnus rubra red alder tree 80 35 very fast deciduous, 99 999 broadleaf Amalanchier alnifolia serviceberry / saskatoon arborescent 15 8 medium deciduous, shrub 99 99 broadleaf Arbutus menziesii Pacific madrone tree 50 50 very slow evergreen, 99 9 broadleaf Arctostaphylos uva-ursi kinnikinnick low creeping 0.5 mat- fast evergreen, shrub forming 999 broadleaf Berberis aquifolium tall Oregon-grape shrub 8 3 medium evergreen, (Mahonia aquilfolium) 99 99 broadleaf Berberis nervosa low Oregon-grape low shrub 2 3 medium evergreen, (Mahonia aquifolium) 99 9 99 broadleaf Cornus nuttalli Pacific flowering dogwood tree 40 20 medium deciduous, 99 99 broadleaf Cornus sericea red-osier dogwood shrub 15 thicket- very fast deciduous, forming 99 9 9 9 broadleaf Corylus cornuta var. californica California hazel / beaked shrub 20 15 fast deciduous, hazelnut 99 9 9 broadleaf -

Dioscorides De Materia Medica Pdf
Dioscorides de materia medica pdf Continue Herbal written in Greek Discorides in the first century This article is about the book Dioscorides. For body medical knowledge, see Materia Medica. De materia medica Cover of an early printed version of De materia medica. Lyon, 1554AuthorPediaus Dioscorides Strange plants RomeSubjectMedicinal, DrugsPublication date50-70 (50-70)Pages5 volumesTextDe materia medica in Wikisource De materia medica (Latin name for Greek work Περὶ ὕλης ἰατρικῆς, Peri hul's iatrik's, both means about medical material) is a pharmacopeia of medicinal plants and medicines that can be obtained from them. The five-volume work was written between 50 and 70 CE by Pedanius Dioscorides, a Greek physician in the Roman army. It was widely read for more than 1,500 years until it supplanted the revised herbs during the Renaissance, making it one of the longest of all natural history books. The paper describes many drugs that are known to be effective, including aconite, aloe, coloxinth, colocum, genban, opium and squirt. In all, about 600 plants are covered, along with some animals and minerals, and about 1000 medicines of them. De materia medica was distributed as illustrated manuscripts, copied by hand, in Greek, Latin and Arabic throughout the media period. From the sixteenth century, the text of the Dioscopide was translated into Italian, German, Spanish and French, and in 1655 into English. It formed the basis of herbs in these languages by such people as Leonhart Fuchs, Valery Cordus, Lobelius, Rembert Dodoens, Carolus Klusius, John Gerard and William Turner. Gradually these herbs included more and more direct observations, complementing and eventually displacing the classic text.