Supplementary Materials 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synergistic Effect of the Herbal Mixture C5E on Gemcitabine Treatment in PANC‑1 Cells
MOLECULAR MEDICINE REPORTS 23: 315, 2021 Synergistic effect of the herbal mixture C5E on gemcitabine treatment in PANC‑1 cells PYO JUNE PAK1*, DONG GUN LEE1*, JI HYUN SUNG2, SEUNG HYUN JUNG3, TAE‑YOUNG HAN4, SUNG HYO PARK1 and NAMHYUN CHUNG1 1Department of Biosystems and Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul 02841; 2Flow Cytometry Core Facility, Biomedical Research Institute, Seoul National University Hospital, Seoul 03082; 3School of Oriental Medicine, Dongguk University, Ilsan 10326; 4BanryongInsu Herb Clinic, Seoul 06099, Republic of Korea Received April 1, 2020; Accepted September 28, 2020 DOI: 10.3892/mmr.2021.11954 Abstract. The present study aimed to determine the anticancer the treatment with either C5E or gemcitabine alone. As the effect of the herbal mixture extract C5E in the pancreatic cancer co‑treatment with gemcitabine and C5E was more effective cell line, PANC‑1, in the absence or presence of gemcitabine than each individual treatment, the present study suggested treatment, a chemotherapeutic drug used for the treatment of that the combined treatment may exhibit synergistic effects in pancreatic cancer. The anticancer effects of C5E, gemcitabine PANC‑1 cells. and C5E plus gemcitabine in PANC‑1 cells following 72 h of treatment were investigated. The effect of each treatment on Introduction cell cycle arrest, apoptosis and the proportion of side popula‑ tion (SP) cells was determined using flow cytometric analysis Pancreatic cancer is a fatal disease, representing the fourth following propidium iodide (PI), Annexin V‑FITC/PI double leading cause of cancer‑related deaths worldwide. Only a few staining and Hoechst 33342 staining, respectively. -

The Rise of Traditional Chinese Medicine and Its Materia Medica A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Bath Research Portal Citation for published version: Williamson, EM, Lorenc, A, Booker, A & Robinson, N 2013, 'The rise of traditional Chinese medicine and its materia medica: a comparison of the frequency and safety of materials and species used in Europe and China', Journal of Ethnopharmacology, vol. 149, no. 2, pp. 453-62. https://doi.org/10.1016/j.jep.2013.06.050 DOI: 10.1016/j.jep.2013.06.050 Publication date: 2013 Document Version Early version, also known as pre-print Link to publication University of Bath General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 13. May. 2019 Journal of Ethnopharmacology 149 (2013) 453–462 Contents lists available at ScienceDirect Journal of Ethnopharmacology journal homepage: www.elsevier.com/locate/jep The rise of traditional Chinese medicine and its materia medica: A comparison of the frequency and safety of materials and species used in Europe and China Elizabeth M. Williamson a,n, Ava Lorenc b,nn, Anthony Booker c, Nicola Robinson b a University of Reading School -

Network Pharmacology and Traditional Chinese Medicine: Devel- Opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1*
ISSN: 2377-3634 Lu et al. Int J Diabetes Clin Res 2017, 4:077 DOI: 10.23937/2377-3634/1410077 Volume 4 | Issue 2 International Journal of Open Access Diabetes and Clinical Research REVIEW ARTICLE Network Pharmacology and Traditional Chinese Medicine: Devel- opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1* 1College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China 2College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China Check for 3College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China updates *Corresponding author: Yitao Chen, MD, College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected]; Changyu Li, MD, College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected] Abstract Research and Development Dilemma for Anti- Diabetes Drugs Partly due to the failure of single-target drugs, diabetes mel- litus, a chronic metabolic disease with complex pathogene- Type 2 Diabetes Mellitus (T2DM), generally agreed sis and long-term medication requirements, is increasing in to be caused by insulin resistance and/or insulin defi- prevalence worldwide and urgently needs multi-component and multi-target treatments. Traditional Chinese herbs are ciency, constitutes almost 95 percent of all diabetes the principal drug of Chinese medicine, which is effective cases [4]. Because of the pathogenesis, the mainstre- against diabetes. However, Chinese herbs’ mechanism of am anti-diabetic drugs are insulin secretagogues (sul- action is difficult to elucidate due to its multiple components phonylureas and meglitinide analogues), insulin sensi- and multi-target effects. -

Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Publications 1-18-2012 Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats Zhiying Dou Tianjin University of Traditional Chinese Medicine Kefeng Li Michigan Technological University Ping Wang Tianjin University of Traditional Chinese Medicine Liu Cao Tianjin University of Traditional Chinese Medicine Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Recommended Citation Dou, Z., Li, K., Wang, P., & Cao, L. (2012). Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules, 17(1), 951-970. http://doi.org/10.3390/molecules17010951 Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/1969 Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Molecules 2012, 17, 951-970; doi:10.3390/molecules17010951 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats Zhiying Dou 1,*, Kefeng Li 2, Ping Wang 1 and Liu Cao 1 1 College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China 2 Department of Biological Sciences, Michigan Technological University, Houghton, MI 49931, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-22-5959-6235. Received: 29 November 2011; in revised form: 5 January 2012 / Accepted: 9 January 2012 / Published: 18 January 2012 Abstract: Vinegar and wine processing of medicinal plants are two traditional pharmaceutical techniques which have been used for thousands of years in China. -

Agaricus Blazei Or Royal Sun Agaric, Inonotus Obliquus Or Chaga, and Ganoderma Lucidum Or Reishi
VOLUME 56: 4 July-August 2016 www.namyco.org Spaces Still Available for NAMA 2016 SHENANDOAH FORAY! There are still slots available for NAMA’s 2016 foray this September 8-11 in Front Royal, VA. Don’t miss out on this unique foray -- sign up today!* Exciting partnership with Shenandoah National Park. We are thrilled that many of this year’s field trips will be in Shenandoah National Park, authorized under a special research permit and “Bioblitz” designation. This gives NAMA members a unique opportunity to pick mushrooms in the park and contribute to a better understanding of the park’s mycoflora. We really hope you’ll join in on this project. Fantastic Faculty. As you know, field trips are only a part of the foray: at any given point on Friday and Saturday there also will be multiple presentations and workshops running. Speakers and workshop leaders will include: • Denis Benjamin • Susan Hopkins • Gary Lincoff • Alan and Arleen Bessette • Mark Jones • Brian Looney • Walt Sturgeon • Catherine Aime • Jay Justice • Shannon Nix • Rod Tulloss • Michael Castellano • Ryan Kepler • Conrad Schoch • Debbie Viess • Tradd Cotter • Patrick Leacock • Ann and Rob Simpson • Rytas Vilgalys • Roy Halling • James Lendemer • Dorothy Smullen You can read more about the faculty, workshops and walks (and see the great foray tshirt!) on the NAMA web- site (http://namyco.org/nama_shenandoah_foray.php). *To register go to http://mms.namyco.org/members/evr/ reg_event.php?orgcode=NAMA&evid=7001739. Great Location. The foray location is just 15 minutes away from Front Royal, VA, the northern gateway to Shenan- doah National Park. -

Genus Species/Common Names Report Genus/Species Common Name
Genus Species/Common Names Report Genus/Species Common Name Abeliophyllum Distichum White-forsythia Abelmoschus Esculentus Okra Abelmoschus Manihot Manioc-hibiscus Sunset-hibiscus Abies Alba European Silver Fir Silver Fir White Fir Abies Balsamea American Silver Fir Balm of Gilead Balsam Canada Balsam Fir Eastern Fir Abies Concolor Colorado Fir Colorado White Fir Silver Fir White Fir Abies Grandis Giant Fir Grand Fir Lowland Fir Lowland White Fir Silver Fir White Fir Yellow Fir Abies Homolepis Nikko Fir Abies Koreana Korean Fir Abies Pectinata Silver Fir Abies Sachalinensis Sakhalin Fir Abies Sibirica Siberian Fir Abies Veitchii Christmastree Veitch Fir Thursday, January 12, 2017 Page 1 of 229 Genus Species/Common Names Report Genus/Species Common Name Abies Veitchii Veitch's Silver Fir Abronia Villosa Desert Sand-verbena Abrus Fruticulosus No common names identified Abrus Precatorius Coral-beadplant Crab's-eye Indian-licorice Jequirity Jequirity-bean Licorice-vine Love-bean Lucky-bean Minnie-minnies Prayer-beads Precatory Precatory-bean Red-beadvine Rosary-pea Weatherplant Weathervine Acacia Arabica Babul Acacia Egyptian Acacia Indian Gum-arabic-tree Scented-thorn Thorn-mimosa Thorny Acacia Acacia Catechu Black Cutch Catechu Acacia Concinna Soap-pod Acacia Dealbata Mimosa Silver Wattle Acacia Decurrens Green Wattle Acacia Farnesiana Cassie Huisache Thursday, January 12, 2017 Page 2 of 229 Genus Species/Common Names Report Genus/Species Common Name Acacia Farnesiana Opopanax Popinac Sweet Acacia Acacia Mearnsii Black Wattle Tan Wattle -

NRDC: Generally Recognized As Secret
NRDC Report April 2014 Generally Recognized as Secret: Chemicals Added to Food in the United States Tom Neltner, J.D., Maricel Maffini, Ph.D. Natural Resources Defense Council In April 2014, the Natural Resources Defense Council (NRDC) released a report raising concerns about a loophole in the Food Additives Amendment of 1958 for substances designated by food manufacturers as “generally recognized as safe” (GRAS). The report identified 56 companies that appeared to market 275 chemicals for use in food based on undisclosed GRAS safety determinations. For each chemical we identified in this study, we did not find evidence that FDA had cleared them for use in food. The 1958 law exempted from the formal, extended FDA approval process common food ingredients like vinegar and vegetable oil whose use qualifies as GRAS. It may have appeared reasonable at the time, but that exemption has been stretched into a loophole that has swallowed the law. The exemption allows manufacturers to make safety determinations that the uses of their newest chemicals in food are safe without notifying the FDA. The agency’s attempts to limit these undisclosed GRAS determinations by asking industry to voluntarily inform the FDA about their chemicals are insufficient to ensure the safety of our food in today’s global marketplace with a complex food supply. Furthermore, no other developed country in the world has a system like GRAS to provide oversight of food ingredients. In Table 1 and 2 of the report, NRDC identified the 56 companies and the number of chemicals that each company appeared to market as GRAS without FDA clearance. -

Syzygites Megalocarpus (Mucorales, Zygomycetes) in Illinois
Transactions of the Illinois State Academy of Science received 12/8/98 (1999), Volume 92, 3 and 4, pp. 181-190 accepted 6/2/99 Syzygites megalocarpus (Mucorales, Zygomycetes) in Illinois R. L. Kovacs1 and W. J. Sundberg2 Department of Plant Biology, Mail Code 6509 Southern Illinois University at Carbondale Carbondale, Illinois 62901-6509 1Current Address: Salem Academy; 942 Lancaster Dr. NE; Salem, OR 97301 2Corresponding Author ABSTRACT Syzygites megalocarpus Ehrenb.: Fr. (Mucorales, Zygomycetes), which occurs on fleshy fungi and was previously unreported from Illinois, has been collected from five counties- -Cook, Gallatin, Jackson, Union, and Williamson. In Illinois, S. megalocarpus occurs on 23 species in 18 host genera. Fresh host material collected in the field and appearing uninfected can develop S. megalocarpus colonies after incubation in the laboratory. The ability of S. megalocarpus to colonize previously uninfected hosts was demonstrated by inoculation studies in the laboratory. Because the known distribution of potential hosts in Illinois is much broader than documented here, further attention to S. megalocarpus should more fully elucidate the host and geographic ranges of this Zygomycete in the state. Using light and scanning electron microscopy, the heretofore unmeasured warts on the zygosporangium were 4-6 µm broad and 5-8 µm high, providing additional informa- tion for circumscription of this genus. INTRODUCTION Syzygites (Mucorales, Zygomycetes) is a presumptive mycoparasite that occurs on fleshy fungi (Figs. 1-2) and contains a single species, S. megalocarpus Ehrenb.: Fr. (Hesseltine 1957). It is homothallic and forms erect sporangiophores which are dichotomously branched and bear columellate, multispored sporangia at their apices (Fries 1832, Hes- seltine 1957, Benny and O'Donnell 1978, O'Donnell 1979). -

First Cultivation of Agaricus Flocculosipes and a Novel Thai Strain of A
Mycosphere 5 (6): 814–820 (2014) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2014 Online Edition Doi 10.5943/mycosphere/5/6/11 First cultivation of Agaricus flocculosipes and a novel Thai strain of A. subrufescens Thongklang N 1, 2, Sysouphanthong P 3, Callac P 4 and Hyde KD 1,2 1School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 2Institute of Excellence in Fungal Research, and School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 3Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, China 4UR 1264, Mycologie et Sécurité des Aliments, 33883 Villenave d’ Ornon, France Thongklang N, Sysouphanthong P, Callac P, Hyde KD 2014 – First cultivation of Agaricus flocculosipes and a novel Thai strain of A. subrufescens. Mycosphere 5(6), 814–820, Doi 10.5943/mycosphere/5/6/11 Abstract Agaricus flocculosipes and A. subrufescens are edible species that belong to section Arvenses of the genus Agaricus. Agaricus subrufescens (almond mushroom) is known to produce bioactive compounds with medicinal properties, such as anti-cancer and anti-tumor activity and fruiting bodies are also edible and nutritious. Agaricus subrufescens is presently cultivated in Brazil, China, Japan, Taiwan and some European countries for use as foods and nutraceuticals. Agaricus flocculosipes is a newly described species currently known only from Thailand, Mayotte Island and China. Species of Agaricus have high potential for cultivation as many species are edible and have medicinal properties. Herein we report the first cultivation of A. flocculosipes and a Thai strain of A. -

URAT1 for J Ethnopharmacol Figures2
O O O O O O O O coumarin 7-methoxycoumarin OH HO osthol O O O O HO O O 4-hydroxycoumarin 6-hydroxycoumarin umbelliferone HO O HO O O HO O O HO O O HO O O OH OH daphnetin esculetin fraxetin osthenol OH O O OH O O O O O bergaptol bergamottin geraniol Figure S1. Chemical structures of the compounds used as samples in this study. Original ABC F GED Original IH KJ L NM Figure S2. TLC pattern of the fractions collected from the hexane crude fraction. The hexane crude fraction (3.0 g) was applied to open silica gel chromatography eluted with hexane:acetone (8:2), and the fractions A–N were collected. Each fraction (3 µg) was spotted to TLC plate, spread out with hexane:acetone (8:2), and colored with anisaldehyde reagent. “Original” means the original hexane crude fraction. Large single spot in the fraction F is osthol. Table S1. The origin, distributer name, lot number of the sample, and the ratio yielded of crude drugs used. Latin name of crude drug Origin Distributera) Lot # Ratio yielded (%)b) Achyranthis Radix The dried root of Achyranthes bidentata Blume Tsumura 22026591 9.4 Akebiae Caulis The dried climbing stem of Akebia quinata Decaisne Tsumura 23006161 4.2 Alismatis Rhizoma The dried tuber of Alisma plantago-aquatica subsp. orientale (Sampaio) Sampaio Tsumura 22043631 6.8 Alpiniae Officinari Rhizoma The dried rhizome of Alpinia officinarum Hance Tsumura 23011641 9.0 Amomi Semen The dried seed mass of Amomum villosum var. xanthioides (Wall. ex Baker) T.L.Wu & S.J.Chen Tsumura 22042491 1.2 Anemarrhenae Rhizoma The dried rhizome of Anemarrhena asphodeloides Bunge Tsumura 23005341 16.8 Angelicae Dahuricae Radix The dried root of Angelica dahurica Benth. -

Fatty Acids and Stable Isotope Ratios in Shiitake Mushrooms
foods Article Fatty Acids and Stable Isotope Ratios in Shiitake Mushrooms (Lentinula edodes) Indicate the Origin of the Cultivation Substrate Used: A Preliminary Case Study in Korea 1, 1, 2 2 3 Ill-Min Chung y, So-Yeon Kim y, Jae-Gu Han , Won-Sik Kong , Mun Yhung Jung and Seung-Hyun Kim 1,* 1 Department of Crop Science, College of Sanghuh Life Science, Konkuk University, Seoul 05029, Korea; [email protected] (I.-M.C.); [email protected] (S.-Y.K.) 2 National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumseong 27709, Korea; [email protected] (J.-G.H.); [email protected] (W.-S.K.) 3 Department of Food Science and Biotechnology, Graduate School, Woosuk University, Wanju-gun 55338, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-02-2049-6163; Fax: +82-02-455-1044 These authors contributed equally to this study. y Received: 22 July 2020; Accepted: 28 August 2020; Published: 1 September 2020 Abstract: Shiitake mushroom (Lentinula edodes) is commonly consumed worldwide and is cultivated in many farms in Korea using Chinese substrates owing to a lack of knowledge on how to prepare sawdust-based substrate blocks (bag cultivation). Consequently, issues related to the origin of the Korean or Chinese substrate used in shiitake mushrooms produced using bag cultivation have been reported. Here, we investigated differences in fatty acids (FAs) and stable isotope ratios (SIRs) in shiitake mushrooms cultivated using Korean and Chinese substrates under similar conditions (strain, temperature, humidity, etc.) and depending on the harvesting cycle. The total FA level decreased significantly by 5.49 mg g 1 as the harvesting cycle increased (p < 0.0001); however, no differences · − were found in FAs between shiitake mushrooms cultivated using Korean and Chinese substrates. -
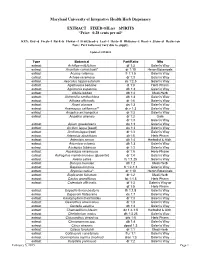
SPIRITS *Price: 0.28 Cents Per Ml*
Maryland University of Integrative Health Herb Dispensary EXTRACT FIXED OIL(s) SPIRITS *Price: 0.28 cents per ml* KEY: Dry=d Fresh=f Bark=b Flower=f Fruit/Seed=s Leaf=l Herb=H Rhizome=z Root=r Stem=st Resin=rsn Note: Part ratio may vary due to supply. Updated 1/29/2015 Type Botanical Part/Ratio Mfg extract Achillea millefolium df 1:3 Galen’s Way extract Aconitum carmichaeli* dr 1:10 Heron Botanicals extract Acorus calamus fr 1:1.5 Galen’s Way extract Actaea racemosa dr 1:3 Galen’s Way extract Aesculus hippocastanum ds 1:2.5 Galen’s Way extract Agathosma betulina dl 1:3 Herb Pharm extract Agrimonia eupatoria dh 1:3 Galen’s Way extract Albizia lebbek db 1:2 Medi-Herb extract Alchemilla xanthochlora dh 1:3 Galen’s Way extract Althaea officinalis dr 1:6 Galen’s Way extract Ammi visnaga ds 1:3 Galen’s Way extract Anemopsis californica** dr,z 1:3 Galen’s Way extract Angelica archangelica dr 1:3 Galen’s Way extract Angelica sinensis dr 1:2 Gaia dr 1:3 Galen’s Way extract Apium graveoloens ds 1:3 Galen’s Way extract Arctium lappa (seed) ds 1:3 Galen’s Way extract Arctium lappa (root) dr 1:3 Galen’s Way extract Artemisia absinthium dh1:5 Herb Pharm extract Artemisia annua dh 1:4 Herbalist & Alch. extract Artemisia vulgaris dh 1:3 Galen’s Way extract Asclepias tuberosa dr 1:3 Galen’s Way extract Asparagus racemosus dr 1:5 Herb-Pharm extract Astragalus membranaceus (glycerite) dr 1:4 Galen’s Way extract Avena sativa fs 1:1.25 Galen’s Way extract Bacopa monnieri dh 1:2 Medi-Herb extract Baptisia tinctoria fr 1:2-1:3 Galen’s Way extract Bryonia cretica* dr 1:10 Heron Botanicals extract Bupleurum falcatum dr 1:2 Medi-Herb extract Cactus grandiflorus fst 1:1.5 Herb Pharm extract Calendula officinalis df 1:3 Galen’s Way or df 1:5 Herb Pharm extract Capsella bursa-pastoris fh 1:1.5 Galen’s Way extract Capsicum frutescens ds 1:7 Galen’s Way extract Caulophyllum thalictroides dr 1:3 Galen’s Way extract Ceanothus americanus dr 1:3 Galen’s Way extract Centella asiatica dh 1:2 Galen’s Way extract Chamaelirium luteum dz 1:4-1:5 Herbalist & Alch.